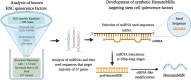
Development of a novel and synthetic HematoMiR technology that broadly modulates quiescence of stem cells and enhances HSC expansion
- PMID: 34971431
- PMCID: PMC11072120
- DOI: 10.1007/s00018-021-04031-1
Development of a novel and synthetic HematoMiR technology that broadly modulates quiescence of stem cells and enhances HSC expansion
Abstract
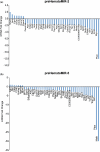
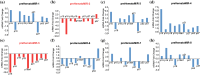
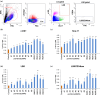
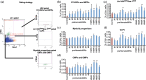
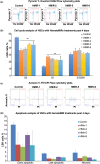
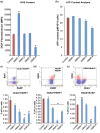
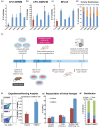
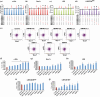
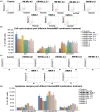
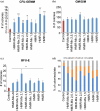
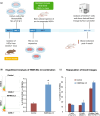
Hematopoietic stem cell (HSCs) transplantation is the primary therapeutic modality used to treat hematopoietic disorders. It centers on the capability of a small quantity of HSCs to repopulate whole blood lineages. Along with limited availability of suitable donors, the need for sufficient number of donor HSCs is still challenging in clinical relevance. This has been addressed by ex vivo HSC expansion albeit with partial success, and thus development of an alternative strategy that could improve HSC expansion is required. To that end, we aimed to build HematoMiR, an oligo-based technology that broadly targets HSC quiescence factors. Here, we show that HematoMiRs and their combinations targeting over 50 factors involved in HSC quiescence could induce robust ex vivo murine and human HSC expansion. In particular, HematoMiR-5 treatment enhanced cell cycle through down-regulation of negative cell cycle regulators in HSCs. HematoMiR-5 treated HSPCs had reduced DNA damage during the course of ex vivo expansion. Moreover, HematoMiR-5 treatment led to sustained HSC self-renewal ability and a low apoptosis rate. In addition, HematoMiR-5 expanded HSCs demonstrated successful engraftment and repopulation capacity in the recipient animals. Furthermore, combinatorial treatments of HematoMiR-2 and 5 allowed vigorous ex vivo HSC expansion. These findings demonstrate that novel and synthetic HematoMiR technology is feasible for HSC ex vivo expansion through the sequence-dependent modulation of numerous HSC quiescence modulators.
Keywords: Engraftment; Ex vivo expansion; HSC self-renewal; RNA interference; Short-RNA.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
All authors declare that they have no conflicts of interest concerning this work.
Figures

Similar articles
-
Comparison of Different Cytokine Conditions Reveals Resveratrol as a New Molecule for Ex Vivo Cultivation of Cord Blood-Derived Hematopoietic Stem Cells.Stem Cells Transl Med. 2015 Sep;4(9):1064-72. doi: 10.5966/sctm.2014-0284. Epub 2015 Jul 9. Stem Cells Transl Med. 2015. PMID: 26160960 Free PMC article.
-
c-Myc Inhibitor 10074-G5 Induces Murine and Human Hematopoietic Stem and Progenitor Cell Expansion and HDR Modulator Rad51 Expression.Curr Cancer Drug Targets. 2019;19(6):479-494. doi: 10.2174/1568009618666180905100608. Curr Cancer Drug Targets. 2019. PMID: 30182856
-
Temporal modulation of calcium sensing in hematopoietic stem cells is crucial for proper stem cell expansion and engraftment.J Cell Physiol. 2020 Dec;235(12):9644-9666. doi: 10.1002/jcp.29777. Epub 2020 May 11. J Cell Physiol. 2020. PMID: 32394484
-
Hematopoietic stem cells: transcriptional regulation, ex vivo expansion and clinical application.Curr Mol Med. 2012 Jan;12(1):34-49. doi: 10.2174/156652412798376125. Curr Mol Med. 2012. PMID: 22082480 Free PMC article. Review.
-
HSC Niche Biology and HSC Expansion Ex Vivo.Trends Mol Med. 2017 Sep;23(9):799-819. doi: 10.1016/j.molmed.2017.07.003. Epub 2017 Aug 8. Trends Mol Med. 2017. PMID: 28801069 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

