Cancer immunotherapy using artificial adjuvant vector cells to deliver NY-ESO-1 antigen to dendritic cells in situ
- PMID: 34971473
- PMCID: PMC8898705
- DOI: 10.1111/cas.15259
Cancer immunotherapy using artificial adjuvant vector cells to deliver NY-ESO-1 antigen to dendritic cells in situ
Abstract
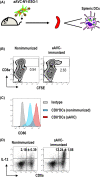
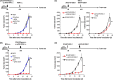
NY-ESO-1 is a cancer/testis antigen expressed in various cancer types. However, the induction of NY-ESO-1-specific CTLs through vaccines is somewhat difficult. Thus, we developed a new type of artificial adjuvant vector cell (aAVC-NY-ESO-1) expressing a CD1d-NKT cell ligand complex and a tumor-associated antigen, NY-ESO-1. First, we determined the activation of invariant natural killer T (iNKT) and natural killer (NK) cell responses by aAVC-NY-ESO-1. We then showed that the NY-ESO-1-specific CTL response was successfully elicited through aAVC-NY-ESO-1 therapy. After injection of aAVC-NY-ESO-1, we found that dendritic cells (DCs) in situ expressed high levels of costimulatory molecules and produced interleukn-12 (IL-12), indicating that DCs undergo maturation in vivo. Furthermore, the NY-ESO-1 antigen from aAVC-NY-ESO-1 was delivered to the DCs in vivo, and it was presented on MHC class I molecules. The cross-presentation of the NY-ESO-1 antigen was absent in conventional DC-deficient mice, suggesting a host DC-mediated CTL response. Thus, this strategy helps generate sufficient CD8+ NY-ESO-1-specific CTLs along with iNKT and NK cell activation, resulting in a strong antitumor effect. Furthermore, we established a human DC-transferred NOD/Shi-scid/IL-2γcnull immunodeficient mouse model and showed that the NY-ESO-1 antigen from aAVC-NY-ESO-1 was cross-presented to antigen-specific CTLs through human DCs. Taken together, these data suggest that aAVC-NY-ESO-1 has potential for harnessing innate and adaptive immunity against NY-ESO-1-expressing malignancies.
Keywords: NKT cell; cancer; cytotoxic T cell; dendritic cell; immunotherapy.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

