Sex-dependent metabolism of ketamine and (2R,6R)-hydroxynorketamine in mice and humans
- PMID: 34971525
- PMCID: PMC9904319
- DOI: 10.1177/02698811211064922
Sex-dependent metabolism of ketamine and (2R,6R)-hydroxynorketamine in mice and humans
Abstract
Background: Ketamine is rapidly metabolized to norketamine and hydroxynorketamine (HNK) metabolites. In female mice, when compared to males, higher levels of (2R,6R;2S,6S)-HNK have been observed following ketamine treatment, and higher levels of (2R,6R)-HNK following the direct administration of (2R,6R)-HNK.
Aim: The objective of this study was to evaluate the impact of sex in humans and mice, and gonadal hormones in mice on the metabolism of ketamine to form norketamine and HNKs and in the metabolism/elimination of (2R,6R)-HNK.
Methods: In CD-1 mice, we utilized gonadectomy to evaluate the role of circulating gonadal hormones in mediating sex-dependent differences in ketamine and (2R,6R)-HNK metabolism. In humans (34 with treatment-resistant depression and 23 healthy controls) receiving an antidepressant dose of ketamine (0.5 mg/kg i.v. infusion over 40 min), we evaluated plasma levels of ketamine, norketamine, and HNKs.
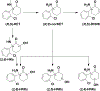
Results: In humans, plasma levels of ketamine and norketamine were higher in males than females, while (2R,6R;2S,6S)-HNK levels were not different. Following ketamine administration to mice (10 mg/kg i.p.), Cmax and total plasma concentrations of ketamine and norketamine were higher, and those of (2R,6R;2S,6S)-HNK were lower, in intact males compared to females. Direct (2R,6R)-HNK administration (10 mg/kg i.p.) resulted in higher levels of (2R,6R)-HNK in female mice. Ovariectomy did not alter ketamine metabolism in female mice, whereas orchidectomy recapitulated female pharmacokinetic differences in male mice, which was reversed with testosterone replacement.
Conclusion: Sex is an important biological variable that influences the metabolism of ketamine and the HNKs, which may contribute to sex differences in therapeutic antidepressant efficacy or side effects.
Keywords: Ketamine; antidepressant; depression; humans; hydroxynorketamine; mice.
Conflict of interest statement
DECLARATION OF COMPETING INTERESTS
RM and CAZ are listed as co-inventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine and other stereoisomeric dehydro- and hydroxylated metabolites of (R,S)-ketamine in the treatment of depression and neuropathic pain. JNH, PZ, RM, CAZ, and TG are listed as co-inventors on patents or patent application for the pharmacology or use of (2R,6R)-hydroxynorketamine, (2S,6S)-hydroxynorketamine, and molecular variants relevant to the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorders. RM, PM, CAZ, and CT have assigned their patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. JNH, PZ and TG have assigned their patent rights to the University of Maryland Baltimore but will share a percentage of any royalties that may be received by the University of Maryland Baltimore.
Figures
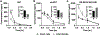

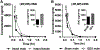


Similar articles
-
Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression.Br J Clin Pharmacol. 2012 Aug;74(2):304-14. doi: 10.1111/j.1365-2125.2012.04198.x. Br J Clin Pharmacol. 2012. PMID: 22295895 Free PMC article. Clinical Trial.
-
(S)-norketamine and (2S,6S)-hydroxynorketamine exert potent antidepressant-like effects in a chronic corticosterone-induced mouse model of depression.Pharmacol Biochem Behav. 2020 Apr;191:172876. doi: 10.1016/j.pbb.2020.172876. Epub 2020 Feb 20. Pharmacol Biochem Behav. 2020. PMID: 32088360
-
Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine.Neuropsychopharmacology. 2020 Aug;45(9):1545-1556. doi: 10.1038/s41386-020-0714-z. Epub 2020 May 17. Neuropsychopharmacology. 2020. PMID: 32417852 Free PMC article.
-
CYP 450 enzymes influence (R,S)-ketamine brain delivery and its antidepressant activity.Neuropharmacology. 2022 Mar 15;206:108936. doi: 10.1016/j.neuropharm.2021.108936. Epub 2021 Dec 26. Neuropharmacology. 2022. PMID: 34965407 Review.
-
The antidepressant potential of (2R,6R)-hydroxynorketamine: A detailed review of pre-clinical findings.Eur J Pharmacol. 2025 Jul 15;999:177604. doi: 10.1016/j.ejphar.2025.177604. Epub 2025 Apr 8. Eur J Pharmacol. 2025. PMID: 40209847 Review.
Cited by
-
Ketamine-induced static and dynamic functional connectivity changes are modulated by opioid receptors and biological sex in rats.Neuropsychopharmacology. 2025 Apr 19. doi: 10.1038/s41386-025-02108-0. Online ahead of print. Neuropsychopharmacology. 2025. PMID: 40253549
-
Psychedelic Drugs in Mental Disorders: Current Clinical Scope and Deep Learning-Based Advanced Perspectives.Adv Sci (Weinh). 2025 Apr;12(15):e2413786. doi: 10.1002/advs.202413786. Epub 2025 Mar 20. Adv Sci (Weinh). 2025. PMID: 40112231 Free PMC article. Review.
-
Ketamine, an Old-New Drug: Uses and Abuses.Pharmaceuticals (Basel). 2023 Dec 21;17(1):16. doi: 10.3390/ph17010016. Pharmaceuticals (Basel). 2023. PMID: 38276001 Free PMC article. Review.
-
The effects of (2R,6R)-hydroxynorketamine on oxycodone withdrawal and reinstatement.Drug Alcohol Depend. 2023 Dec 1;253:110987. doi: 10.1016/j.drugalcdep.2023.110987. Epub 2023 Oct 5. Drug Alcohol Depend. 2023. PMID: 37864957 Free PMC article.
-
Sex dependence of opioid-mediated responses to subanesthetic ketamine in rats.Nat Commun. 2024 Jan 30;15(1):893. doi: 10.1038/s41467-024-45157-7. Nat Commun. 2024. PMID: 38291050 Free PMC article.
References
-
- Adams JD Jr., Baillie TA, Trevor AJ, et al. (1981) Studies on the biotransformation of ketamine. 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spectrom 8(11): 527–538. - PubMed
-
- Barre L, Fournel-Gigleux S, Finel M, et al. (2007) Substrate specificity of the human UDP-glucuronosyltransferase UGT2B4 and UGT2B7. Identification of a critical aromatic amino acid residue at position 33. FEBS J 274(5): 1256–1264. - PubMed
-
- Berman RM, Cappiello A, Anand A, et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4): 351–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

