Survival by age in paediatric and adolescent patients with Hodgkin lymphoma: a retrospective pooled analysis of children's oncology group trials
- PMID: 34971582
- PMCID: PMC8815096
- DOI: 10.1016/S2352-3026(21)00349-5
Survival by age in paediatric and adolescent patients with Hodgkin lymphoma: a retrospective pooled analysis of children's oncology group trials
Abstract
Background: Adolescents with Hodgkin lymphoma have worse disease outcomes than children. Whether these differences persist within clinical trials is unknown. We examined survival, by age, in patients receiving response-adapted therapy for Hodgkin lymphoma on Children's Oncology Group (COG) trials.
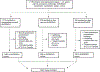
Methods: Patients (aged 1-21 years) diagnosed with classical Hodgkin lymphoma and enrolled between Sept 23, 2002, and Jan 19, 2012, on one of three phase 3 COG trials in the USA and Canada were eligible for inclusion. The three COG trials were defined by risk group according to Ann Arbor stage, B-symptoms, and bulk (AHOD0431 [low risk; NCT00302003], AHOD0031 [intermediate risk; NCT00025259], or AHOD0831 [high risk; NCT01026220]). The outcomes of this study were event-free survival (death, relapse, or subsequent neoplasm) and overall survival. Cox proportional hazards models estimated survival, adjusting for disease and treatment factors both overall and in patients with mixed cellularity or non-mixed cellularity (nodular sclerosing and not-otherwise-specified) disease.
Findings: Of 2155 patients enrolled on the three trials, 1907 (88·4%; 968 [50·8%] male and 939 [49·2%] female; 1227 [64·3%] non-Hispanic White) were included in this analysis. After a median follow-up of 7·4 years (IQR 4·3-10·2), older patients (aged ≥15 years) had worse unadjusted 5-year event-free survival (80% [95% CI 78-83]) than did younger patients (aged <15 years; 86% [83-88]; HR 1·38 [1·11-1·71]; p=0·0038). Older patients also had worse unadjusted 5-year overall survival than did younger patients (96% [95% CI 95-97] vs 99% [98-99]; HR 2·50 [1·41-4·45]; p=0·0012). In patients with non-mixed cellularity histology, older patients had a significantly increased risk of having an event than did younger patients with the same histology (HR 1·32 [1·03-1·68]; p=0·027). Older patients with mixed cellularity had significantly worse 5-year event-free survival than did younger patients in unadjusted (77% [95% CI 65-86] for older patients vs 94% [88-97] for younger patients; HR 2·93 [1·37-6·29]; p=0·0039) and multivariable models (HR 3·72 [1·56-8·91]; p=0·0032). Overall, older patients were more likely to die than younger patients (HR 3·08 [1·49-6·39]; p=0·0025).
Interpretation: Adolescents (≥15 years) treated on COG Hodgkin lymphoma trials had worse event-free survival and increased risk of death compared with children (<15 years). Our findings highlight the need for prospective studies to examine tumour and host biology, and to test novel therapies across the age spectrum.
Funding: National Institutes of Health, St Baldrick's Foundation, and Lymphoma Research Foundation.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

References
-
- Henderson TO, Parsons SK, Wroblewski KE, Chen L, Hong F, Smith SM, et al. Outcomes in adolescents and young adults with Hodgkin lymphoma treated on US cooperative group protocols: An adult intergroup (E2496) and Children’s Oncology Group (COG AHOD0031) comparative analysis. Cancer 2018;124(1):136–44. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

