Stress while lacking of control induces ventral hippocampal autophagic flux hyperactivity and a depression-like behavior
- PMID: 34971825
- PMCID: PMC9795357
- DOI: 10.1016/j.bj.2021.12.008
Stress while lacking of control induces ventral hippocampal autophagic flux hyperactivity and a depression-like behavior
Abstract
Background: Stressed animals may perform depression-like behavior insomuch as stress-provoking blood-brain barrier (BBB) disruption, central immune activation, and autophagic flux changes. This study was undertaken to assess whether adult mice having (executive) vs. lacking (yoke) of behavioral control in otherwise equivalent stress magnitude condition, may display differences in their BBB integrity, ventral hippocampal (VH) interleukin-6 (IL-6) and autophagic flux level and VH-related depression-like behavior. To further understand the causative relation of enhanced autophagic flux and stress-primed depression-like behavior, we assessed the effects of bilateral intra-VH 3-methyladenine (3-MA), an autophagic flux inhibitor, infusion in stressed mice.
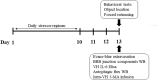
Methods: Adult mice used had comparable genetic background and housing condition. Executive/yoke pairs of mice received a 10-day (1 h/day) footshock stressor regimen. Throughout the regimen, the ongoing footshock was terminated immediately contingent on the executive mouse', while irrelevant to the respective yoke mouse' voluntary behavior, or lasting for 7 s. Each dyad's cage-mate receiving no such regimen served as no stressor controls.
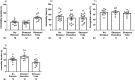
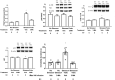
Results: Yoke mice displayed disrupted BBB integrity (escalated Evans blue extravasation and decreased VH ZO-1, claudin-5 expression), increases in VH autophagic flux (increased LC3II/LC3I and decreased p62) and immobility duration in forced swimming test. Most of these indices remained unaltered in executive mice. Administration of 3-MA did not affect immobility duration in control mice, while prevented the increases in immobility duration in yoke mice.
Conclusions: (1) stress susceptibility may be determined by their differences in stress-coping results; (2) VH autophagic flux increase plays a permissive role in priming the stressed animals susceptible to exhibit depression-like behavior.
Keywords: Autophagy; Blood–brain barrier; Executive; Forced swimming; Hippocampus; Yoke.
Copyright © 2021 Chang Gung University. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest None.
Figures

Similar articles
-
NMDA-NO signaling in the dorsal and ventral hippocampus time-dependently modulates the behavioral responses to forced swimming stress.Behav Brain Res. 2016 Jul 1;307:126-36. doi: 10.1016/j.bbr.2016.03.037. Epub 2016 Mar 22. Behav Brain Res. 2016. PMID: 27016428
-
[Effect of Acupuncture Intervention on c-jun N-terminal Kinase Signaling in the Hippocampus in Rats with Forced Swimming Stress].Zhen Ci Yan Jiu. 2016 Feb;41(1):18-23. Zhen Ci Yan Jiu. 2016. PMID: 27141615 Chinese.
-
HSPB8 over-expression prevents disruption of blood-brain barrier by promoting autophagic flux after cerebral ischemia/reperfusion injury.J Neurochem. 2019 Jan;148(1):97-113. doi: 10.1111/jnc.14626. Epub 2018 Dec 13. J Neurochem. 2019. PMID: 30422312
-
Active coping of prenatally stressed rats in the forced swimming test: involvement of the Nurr1 gene.Stress. 2016 Sep;19(5):506-15. doi: 10.1080/10253890.2016.1193147. Epub 2016 Jun 13. Stress. 2016. PMID: 27219004
-
Hippocampal ornithine decarboxylase/spermidine pathway mediates H2S-alleviated cognitive impairment in diabetic rats: Involving enhancment of hippocampal autophagic flux.J Adv Res. 2020 Jun 12;27:31-40. doi: 10.1016/j.jare.2020.06.007. eCollection 2021 Jan. J Adv Res. 2020. PMID: 33318864 Free PMC article. Review.
Cited by
-
Effects of Septin-14 Gene Deletion on Adult Cognitive/Emotional Behavior.Front Mol Neurosci. 2022 Apr 29;15:880858. doi: 10.3389/fnmol.2022.880858. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35571367 Free PMC article.
-
Vulnerability of the Hippocampus to Insults: Links to Blood-Brain Barrier Dysfunction.Int J Mol Sci. 2024 Feb 6;25(4):1991. doi: 10.3390/ijms25041991. Int J Mol Sci. 2024. PMID: 38396670 Free PMC article. Review.
-
Xiaoyaosan Exerts Antidepressant-Like Effect by Regulating Autophagy Involves the Expression of GLUT4 in the Mice Hypothalamic Neurons.Front Pharmacol. 2022 Jun 16;13:873646. doi: 10.3389/fphar.2022.873646. eCollection 2022. Front Pharmacol. 2022. PMID: 35784760 Free PMC article.
-
Small and mighty - microRNAs pulling the strings.Biomed J. 2022 Dec;45(6):851-856. doi: 10.1016/j.bj.2022.11.003. Epub 2022 Nov 26. Biomed J. 2022. PMID: 36442792 Free PMC article.
-
Male Stressed Mice Having Behavioral Control Exhibit Escalations in Dorsal Dentate Adult-Born Neurons and Spatial Memory.Int J Mol Sci. 2023 Jan 19;24(3):1983. doi: 10.3390/ijms24031983. Int J Mol Sci. 2023. PMID: 36768303 Free PMC article.
References
-
- Calderón-Garcidueñas L., Vojdani A., Blaurock-Busch E., Busch Y., Friedle A., Franco-Lira M., et al. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J Alzheimers Dis. 2015;43:1039–1058. - PubMed
-
- Tarantini S., Valcarcel-Ares M.N., Yabluchanskiy A., Tucsek Z., Hertelendy P., Kiss T., et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, Amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018;73:853–863. - PMC - PubMed
-
- Valcarcel-Ares M.N., Tucsek Z., Kiss T., Giles C.B., Tarantini S., Yabluchanskiy A., et al. Obesity in aging exacerbates neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontol A Biol Sci Med Sci. 2019;74:290–298. - PMC - PubMed
-
- Wu Q., Mi Y., Cheng W., Xia C., Zhu D., Du D. Infiltrating T helper 17 cells in the paraventricular nucleus are pathogenic for stress-induced hypertension. Biochem Biophys Res Commun. 2019;515:169–175. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

