Evaluation of serological anti-SARS-CoV-2 chemiluminescent immunoassays correlated to live virus neutralization test, for the detection of anti-RBD antibodies as a relevant alternative in COVID-19 large-scale neutralizing activity monitoring
- PMID: 34971839
- PMCID: PMC8714258
- DOI: 10.1016/j.clim.2021.108918
Evaluation of serological anti-SARS-CoV-2 chemiluminescent immunoassays correlated to live virus neutralization test, for the detection of anti-RBD antibodies as a relevant alternative in COVID-19 large-scale neutralizing activity monitoring
Abstract
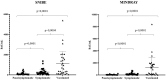
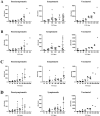
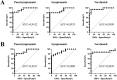
The Spike-Receptor Binding Domain (S-RBD) is considered the most antigenic protein in SARS-CoV-2 and probably the key player in SARS-CoV-2 immune response. Quantitative immunoassays may help establish an anti-RBD Abs threshold as an indication of protective immunity. Since different immunoassays are commercial, the standard reference method for the neutralizing activity is the live Virus Neutralization Test (VNT). In this study, anti-RBD IgG levels were detected with two chemiluminescent immunoassays in paucisymptomatic, symptomatic and vaccinated subjects, and their neutralizing activity was correlated to VNT titer, using SARS-CoV-2 original and British variant strains. Both immunoassays confirmed higher anti-RBD Abs levels in vaccinated subjects. Furthermore, despite different anti-RBD Abs median concentrations between the immunoassays, a strong positive correlation with VNT was observed. In conclusion, although the SARS-CoV-2 immune response heterogeneity, the use of immunoassays can help in large-scale monitoring of COVID-19 samples, becoming a valid alternative to VNT test for diagnostic routine laboratories.
Keywords: Anti-SARS-CoV-2 antibodies; COVID-19; Live virus neutralization test; SARS-CoV-2; Serological immunoassays.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
The Authors declare that they have no conflict of interest.
Figures



Similar articles
-
Serological anti-SARS-CoV-2 neutralizing antibodies association to live virus neutralizing test titers in COVID-19 paucisymptomatic/symptomatic patients and vaccinated subjects.Int Immunopharmacol. 2021 Dec;101(Pt B):108215. doi: 10.1016/j.intimp.2021.108215. Epub 2021 Oct 4. Int Immunopharmacol. 2021. PMID: 34649115 Free PMC article.
-
Correlation of the Commercial Anti-SARS-CoV-2 Receptor Binding Domain Antibody Test with the Chemiluminescent Reduction Neutralizing Test and Possible Detection of Antibodies to Emerging Variants.Microbiol Spectr. 2021 Dec 22;9(3):e0056021. doi: 10.1128/Spectrum.00560-21. Epub 2021 Dec 1. Microbiol Spectr. 2021. PMID: 34851163 Free PMC article.
-
SARS-CoV-2 antibodies: Comparison of three high-throughput immunoassays versus the neutralization test.Eur J Clin Invest. 2021 Jul;51(7):e13573. doi: 10.1111/eci.13573. Epub 2021 May 5. Eur J Clin Invest. 2021. PMID: 33870493 Free PMC article. No abstract available.
-
Recent Developments in SARS-CoV-2 Neutralizing Antibody Detection Methods.Curr Med Sci. 2021 Dec;41(6):1052-1064. doi: 10.1007/s11596-021-2470-7. Epub 2021 Dec 21. Curr Med Sci. 2021. PMID: 34935114 Free PMC article. Review.
-
Rapid lateral flow tests for the detection of SARS-CoV-2 neutralizing antibodies.Expert Rev Mol Diagn. 2021 Apr;21(4):363-370. doi: 10.1080/14737159.2021.1913123. Epub 2021 Apr 12. Expert Rev Mol Diagn. 2021. PMID: 33840347 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Antinucleocapsid Antibody Response of mRNA and Inactivated Virus Vaccines Compared to Unvaccinated Individuals.Vaccines (Basel). 2022 Apr 20;10(5):643. doi: 10.3390/vaccines10050643. Vaccines (Basel). 2022. PMID: 35632399 Free PMC article.
-
Performance Evaluation of RapiSure (EDGC) COVID-19 S1 RBD IgG/Neutralizing Ab Test for the Rapid Detection of SARS-CoV-2 Antibodies.Diagnostics (Basel). 2023 Feb 9;13(4):643. doi: 10.3390/diagnostics13040643. Diagnostics (Basel). 2023. PMID: 36832131 Free PMC article.
-
Overlaid Lateral Flow Immunoassay for the Simultaneous Detection of Two Variant-Specific SARS-CoV-2 Neutralizing Antibodies.Anal Chem. 2024 Apr 9;96(14):5407-5415. doi: 10.1021/acs.analchem.3c05144. Epub 2024 Mar 13. Anal Chem. 2024. PMID: 38478766 Free PMC article.
-
Early Warning Signs for Monitoring Airborne Respiratory Virus Transmission.Int J Environ Res Public Health. 2025 Jul 20;22(7):1151. doi: 10.3390/ijerph22071151. Int J Environ Res Public Health. 2025. PMID: 40724216 Free PMC article. Review.
-
Longitudinal kinetics of RBD+ antibodies in COVID-19 recovered patients over 14 months.PLoS Pathog. 2022 Jun 3;18(6):e1010569. doi: 10.1371/journal.ppat.1010569. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35658051 Free PMC article.
References
-
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020 Mar;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020 Mar 11;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

