Pembrolizumab plus dinaciclib in patients with hematologic malignancies: the phase 1b KEYNOTE-155 study
- PMID: 34972202
- PMCID: PMC8864641
- DOI: 10.1182/bloodadvances.2021005872
Pembrolizumab plus dinaciclib in patients with hematologic malignancies: the phase 1b KEYNOTE-155 study
Abstract
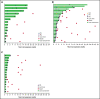
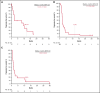
Preclinical data demonstrated that combining an anti-programmed cell death 1 (PD-1) inhibitor with a cyclin-dependent kinase 9 (CDK9) inhibitor provided enhanced antitumor activity with no significant toxicities, suggesting this combination may be a potential therapeutic option. The multicohort, phase 1 KEYNOTE-155 study evaluated the safety and antitumor activity of the PD-1 inhibitor pembrolizumab plus the CDK9 inhibitor dinaciclib in patients with relapsed or refractory (rr) chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL) and multiple myeloma (MM). Patients enrolled were ≥18 years of age with a confirmed diagnosis of CLL, DLBCL, or MM. The study included 2 phases: a dose-evaluation phase to determine dose-limiting toxicities and a signal-detection phase. Patients received pembrolizumab 200 mg every 3 weeks plus dinaciclib 7 mg/m2 on day 1 and 10 mg/m2 on day 8 of cycle 1 and 14 mg/m2 on days 1 and 8 of cycles 2 and later. Primary endpoint was safety, and a key secondary endpoint was objective response rate (ORR). Seventy-two patients were enrolled and received ≥1 dose of study treatment (CLL, n = 17; DLBCL, n = 38; MM, n = 17). Pembrolizumab plus dinaciclib was generally well tolerated and produced no unexpected toxicities. The ORRs were 29.4% (5/17, rrCLL), 21.1% (8/38, rrDLBCL), and 0% (0/17, rrMM), respectively. At data cutoff, all 72 patients had discontinued treatment, 38 (52.8%) because of progressive disease. These findings demonstrate activity with combination pembrolizumab plus dinaciclib and suggest that a careful and comprehensive approach to explore anti-PD-1 and CDK9 inhibitor combinations is warranted. This trial was registered at www.clinicaltrials.gov as NCT02684617.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Version 1.2021. Chronic Lymphocytic leukemia/Small Lymphocytic Lymphoma. Vol. 2020. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

