Diethyl Azelate for the Treatment of Brown Recluse Spider Bite, a Neglected Orphan Indication
- PMID: 34972703
- PMCID: PMC8765177
- DOI: 10.21873/invivo.12679
Diethyl Azelate for the Treatment of Brown Recluse Spider Bite, a Neglected Orphan Indication
Abstract
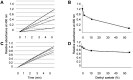
Background/aim: Brown recluse spider bite releases hemolytic and cytotoxic phospholipase D to the wound that may cause necrosis or even death. We examined diethyl azelate (DEA), a plasma membrane fluidizer with a broad range of immunomodulatory activities, as a potential treatment for the brown recluse spider bite.
Materials and methods: Topical DEA was used in emergency to treat brown recluse spider bites in a human subject. We subsequently evaluated the effects of DEA on hemolysis induced by the brown recluse spider venom, recluse recombinant phospholipase D (rPLD), and venoms from honey bee and moccasin snake, and on phospholipase A2 activity in the bee and snake venoms and in human urine.
Results: Topical DEA resolved the consequences of human brown recluse spider envenomation in two weeks. In vitro, DEA inhibited hemolysis caused by the brown recluse spider venom and rPLD and suppressed phospholipase A2 activity in a dose-dependent manner.
Conclusion: DEA is a promising novel therapy for the brown recluse spider bite and perhaps even unrelated envenomations involving PLDs.
Keywords: Azelate; brown recluse; fatty acid ester; spider bite.
Copyright © 2022 International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
EI and RTS are the owners and officers of New Frontier Labs, LLC, the sponsor of all studies described in the manuscript.
Figures




References
-
- Forrester JD, Forrester JA, Tennakoon L, Staudenmayer K. Mortality, hospital admission, and healthcare cost due to injury from venomous and non-venomous animal encounters in the USA: 5-year analysis of the National Emergency Department Sample. Trauma Surg Acute Care Open. 2018;3(1):e000250. doi: 10.1136/tsaco-2018-000250. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
