Effect of dose adjustments on the efficacy and safety of tofacitinib in patients with rheumatoid arthritis: a post hoc analysis of an open-label, long-term extension study (ORAL Sequel)
- PMID: 34973077
- PMCID: PMC8913559
- DOI: 10.1007/s10067-021-05908-z
Effect of dose adjustments on the efficacy and safety of tofacitinib in patients with rheumatoid arthritis: a post hoc analysis of an open-label, long-term extension study (ORAL Sequel)
Abstract
Introduction/objectives: We assess the impact of switching versus staying on the same tofacitinib dose on efficacy and safety in patients with rheumatoid arthritis (RA).
Methods: ORAL Sequel was an open-label, long-term extension study of patients with RA receiving tofacitinib 5 or 10 mg BID for up to 9.5 years. Tofacitinib doses could be switched during the study at investigator discretion. In this post hoc analysis, data from ORAL Sequel were stratified into four groups: 5 → 10 mg BID (Dose-up); 5 mg BID (Stay-on 5); 10 → 5 mg BID (Dose-down); and 10 mg BID (Stay-on 10). Efficacy assessments over 12 months included: change from baseline in 4-component Disease Activity Score in 28 joints, erythrocyte sedimentation rate (DAS28), and DAS28 minimum clinically important difference, remission, and low disease activity (LDA) rates. Safety was assessed for the study duration.
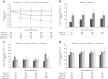
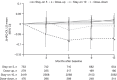
Results: Generally, DAS28 improvements and minimum clinically important difference rates were significantly greater (p < 0.05) in Dose-up versus Stay-on 5 up to month 12. DAS28 remission rates were significantly greater in Dose-up versus Stay-on 5 at month 12. Change from baseline in DAS28 was similar in Dose-down and Stay-on 10. No significant differences in DAS28 LDA rates were observed between groups. Safety data were similar overall across the four groups.
Conclusion: In patients with RA receiving open-label tofacitinib, this analysis found that some benefited from increasing dose from 5 to 10 mg BID and did not find that reducing dose from 10 to 5 mg BID affected efficacy or that dose switching in either direction affected safety.
Study registration: ClinicalTrials.gov number NCT00413699. Registered December 20, 2006. https://clinicaltrials.gov/ct2/show/NCT00413699 Key Points • This post hoc analysis of data from the long-term extension study, ORAL Sequel, assessed the impact of dose switching between tofacitinib 5 and 10 mg twice daily (BID), at the investigator's discretion, on efficacy and safety in patients with rheumatoid arthritis (RA). • Dosing up from tofacitinib 5 to 10 mg BID was associated with improved efficacy up to 12 months versus staying on 5 mg BID, and dosing down from 10 to 5 mg BID was not generally associated with a significant loss of efficacy. • Safety outcomes were generally consistent across dose groups and did not change markedly after switching dose in either direction. • These findings can help to inform physicians on what may be expected in terms of efficacy and safety when adjusting tofacitinib dose according to clinical need. The recommended tofacitinib dosage for the treatment of RA in most jurisdictions is 5 mg BID.
Keywords: Dosing; Post hoc analysis; Rheumatoid arthritis; Tofacitinib.
© 2021. The Author(s).
Conflict of interest statement
RBM has received consulting fees or other remuneration from Pfizer Inc. HS-K has received consulting fees or other remuneration from Pfizer Inc. DEF has received consulting fees or other remuneration from AbbVie, Amgen, Boehringer Ingelheim, Corbus, NIH, Novartis, Pfizer Inc, and Roche-Genentech. SBC has received consulting fees or other remuneration from AbbVie, Amgen, Boehringer Ingelheim, Gilead, Merck, and Pfizer Inc. KK and LW are employees and shareholders of Pfizer Inc. TK is an employee of Pfizer AG and shareholder of Pfizer Inc. JvK has received consulting fees or other remuneration from AbbVie, Bristol-Myers Squibb, Menarini, Pfizer Inc, and Sanofi.
Figures
References
-
- Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. - PubMed
-
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

