Efficacy and safety of esketamine nasal spray by sex in patients with treatment-resistant depression: findings from short-term randomized, controlled trials
- PMID: 34973081
- PMCID: PMC8921149
- DOI: 10.1007/s00737-021-01185-6
Efficacy and safety of esketamine nasal spray by sex in patients with treatment-resistant depression: findings from short-term randomized, controlled trials
Abstract
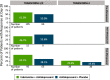
The objective of this analysis was to determine if there are sex differences with esketamine for treatment-resistant depression (TRD). Post hoc analyses of three randomized, controlled studies of esketamine in patients with TRD (TRANSFORM-1, TRANSFORM-2 [18-64 years], TRANSFORM-3 [≥ 65 years]) were performed. In each 4-week study, adults with TRD were randomized to esketamine or placebo nasal spray, each with a newly initiated oral antidepressant. Change from baseline to day 28 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score was assessed by sex in pooled data from TRANSFORM-1/TRANSFORM-2 and separately in data from TRANSFORM-3 using a mixed-effects model for repeated measures. Use of hormonal therapy was assessed in all women, and menopausal status was assessed in women in TRANSFORM-1/TRANSFORM-2. Altogether, 702 adults (464 women) received ≥ 1 dose of intranasal study drug and antidepressant. Mean MADRS total score (SD) decreased from baseline to day 28, more so among patients treated with esketamine/antidepressant vs. antidepressant/placebo in both women and men: TRANSFORM-1/TRANSFORM-2 women-esketamine/antidepressant -20.3 (13.19) vs. antidepressant/placebo -15.8 (14.67), men-esketamine/antidepressant -18.3 (14.08) vs. antidepressant/placebo -16.0 (14.30); TRANSFORM-3 women-esketamine/antidepressant -9.9 (13.34) vs. antidepressant/placebo -6.9 (9.65), men-esketamine/antidepressant -10.3 (11.96) vs. antidepressant/placebo -5.5 (7.64). There was no significant sex effect or treatment-by-sex interaction (p > 0.35). The most common adverse events in esketamine-treated patients were nausea, dissociation, dizziness, and vertigo, each reported at a rate higher in women than men. The analyses support antidepressant efficacy and overall safety of esketamine nasal spray are similar between women and men with TRD. The TRANSFORM studies are registered at clinicaltrials.gov (identifiers: NCT02417064 (first posted 15 April 2015; last updated 4 May 2020), NCT02418585 (first posted 16 April 2015; last updated 2 June 2020), and NCT02422186 (first posted 21 April 2015; last updated 29 September 2021)).
Keywords: Esketamine; Sex; Treatment-resistant depression; s-Ketamine.
© 2021. Janssen Research & Development, LLC.
Conflict of interest statement
Robyn R. Jones, Kimberly Cooper, Ella Daly, Carla M. Canuso, and Susan Nicholson are employees of Janssen Research & Development, LLC and hold company equity. Dr. Marlene Freeman reports Investigator-Initiated Trials/Research: JayMac, Sage; Advisory Boards: Eliem, Sage; Independent Data Safety and Monitoring Committee: Janssen (Johnson & Johnson), Novartis; Steering Committee for Educational Activities: Medscape; and Educational Activities: WebMD. Dr. Freeman is an employee of Massachusetts General Hospital, and works with the MGH National Pregnancy Registry [Current Registry Sponsors: Teva (2018-present), Alkermes, Inc. (2016-present); Otsuka America Pharmaceutical, Inc. (2008-present); Forest/Actavis (2016-present); Sunovion Pharmaceuticals, Inc. (2011-present)]. As an employee of MGH, Dr. Freeman works with the MGH CTNI, which has had research funding from multiple pharmaceutical companies and NIMH. Dr. Susan Kornstein reports Research Support: Pfizer, Allergan, Marinus, Takeda, Palatin, National Science Foundation; Consulting/Advisory Boards: Pfizer, Marinus, Sage, Acadia, Sunovion, Shire, Allergan, Palatin, AbbVie; and Royalties: Guilford Press.
Figures


References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 5th edition: DSM-5. Arlington, TX: American Psychiatric Publishing; 2013.
-
- Berlanga C, Flores-Ramos M (2006) Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J Affect Disord 95(1–3):119–123. 10.1016/j.jad.2006.04.029 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

