A Multi‑Center, Open‑Label, Single‑Arm Trial to Evaluate the Efficacy, Pharmacokinetics, and Safety and Tolerability of IGSC 20% in Subjects with Primary Immunodeficiency
- PMID: 34973143
- PMCID: PMC9016006
- DOI: 10.1007/s10875-021-01181-6
A Multi‑Center, Open‑Label, Single‑Arm Trial to Evaluate the Efficacy, Pharmacokinetics, and Safety and Tolerability of IGSC 20% in Subjects with Primary Immunodeficiency
Erratum in
-
Correction to: A Multi-center, Open-Label, Single-Arm Trial to Evaluate the Efficacy, Pharmacokinetics, and Safety and Tolerability of IGSC 20% in Subjects with Primary Immunodeficiency.J Clin Immunol. 2022 Apr;42(3):512-513. doi: 10.1007/s10875-022-01219-3. J Clin Immunol. 2022. PMID: 35174440 Free PMC article. No abstract available.
Abstract
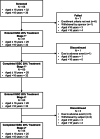
Purpose: The purpose of this phase 3 study was to evaluate the efficacy, pharmacokinetics (PK), and safety of Immune Globulin Subcutaneous (Human), 20% Caprylate/Chromatography Purified (IGSC 20%) in patients with primary immunodeficiency (PI).
Methods: Immunoglobulin treatment-experienced subjects with PI received 52 weeks of IGSC 20% given weekly at the same dose as the subject's previous IgG regimen (DAF 1:1); the minimum dose was 100 mg/kg/week. The primary endpoint was serious bacterial infections (SBIs [null vs alternative hypothesis: SBI rate per person per year ≥ 1 vs < 1]). IgG subclasses and specific pathogen antibody levels were also measured.
Results: Sixty-one subjects (19 children [≤ 12 years], 10 adolescents [> 12-16 years], and 32 adults) were enrolled. The rate of SBIs per person per year was 0.017. The 1-sided 99% upper confidence limit was 0.036 (< 1), and the null hypothesis was rejected. The rate of hospitalization due to infection per person per year was 0.017 (2-sided 95% confidence interval: 0.008-0.033) overall. The mean trough total IgG concentrations were comparable to the previous IgG replacement regimen. The average of the individual mean trough ratios (IGSC 20%:previous regimen) was 1.078 (range: 0.83-1.54). The average steady-state mean trough IgG concentrations were 947.64 and 891.37 mg/dL, respectively. Seven subjects had serious treatment-emergent adverse events (TEAEs); none was drug-related. The rate of all TEAEs, including local infusion site reactions, during 3045 IGSC 20% infusions was 0.135. Most TEAEs were mild or moderate.
Conclusions: IGSC 20% demonstrated efficacy and good safety and tolerability in subjects with PI.
Keywords: 20% immunoglobulin; GTI1503; Primary immunodeficiency; immunoglobulin replacement therapy; subcutaneous.
© 2021. The Author(s).
Conflict of interest statement
MQ, RG, CH, EM, JP, AS, and JL are employed by Grifols Bioscience Research Group, maker of IGSC 20%. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

