Lamina cribrosa vessel and collagen beam networks are distinct
- PMID: 34973204
- PMCID: PMC8923914
- DOI: 10.1016/j.exer.2021.108916
Lamina cribrosa vessel and collagen beam networks are distinct
Abstract
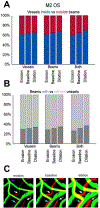
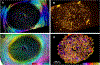
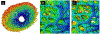
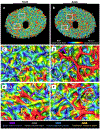
Our goal was to analyze the spatial interrelation between vascular and collagen networks in the lamina cribrosa (LC). Specifically, we quantified the percentages of collagen beams with/without vessels and of vessels inside/outside of collagen beams. To do this, the vasculature of six normal monkey eyes was labeled by perfusion post-mortem. After enucleation, coronal cryosections through the LC were imaged using fluorescence and polarized light microscopy to visualize the blood vessels and collagen beams, respectively. The images were registered to form 3D volumes. Beams and vessels were segmented, and their spatial interrelationship was quantified in 3D. We found that 22% of the beams contained a vessel (range 14%-32%), and 21% of vessels were outside beams (13%-36%). Stated differently, 78% of beams did not contain a vessel (68%-86%), and 79% of vessels were inside a beam (64%-87%). Individual monkeys differed significantly in the fraction of vessels outside beams (p < 0.01 by linear mixed effect analysis), but not in the fraction of beams with vessels (p > 0.05). There were no significant differences between contralateral eyes in the percent of beams with vessels and of vessels outside beams (p > 0.05). Our results show that the vascular and collagenous networks of the LC in monkey are clearly distinct, and the historical notions that each LC beam contains a vessel and all vessels are within beams are inaccurate. We postulate that vessels outside beams may be relatively more vulnerable to mechanical compression by elevated IOP than are vessels shielded inside of beams.
Keywords: Collagen; Lamina cribrosa; Morphology; Optic nerve head; Vasculature.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures









References
-
- Beck RW, Savino PJ, Repka MX, Schatz NJ, Sergott RC. Optic disc structure in anterior ischemic optic neuropathy. Ophthalmology. 1984;91(11):1334–1337. - PubMed
-
- Sommer A Glaucoma risk factors observed in the Baltimore Eye Survey. Curr Opin Ophthalmol. 1996;7(2):93–98. - PubMed
-
- Rizzo JF 3rd. Unraveling the Enigma of Nonarteritic Anterior Ischemic Optic Neuropathy. J Neuroophthalmol. 2019;39(4):529–544. - PubMed
-
- Hayreh SS. Inter-individual variation in blood supply of the optic nerve head. Doc Ophthalmol. 1985;59(3):217–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

