A novel PAK4 inhibitor suppresses pancreatic cancer growth and enhances the inhibitory effect of gemcitabine
- PMID: 34973571
- PMCID: PMC8724943
- DOI: 10.1016/j.tranon.2021.101329
A novel PAK4 inhibitor suppresses pancreatic cancer growth and enhances the inhibitory effect of gemcitabine
Abstract
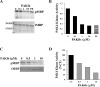
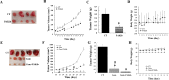
Over 95% of Pancreatic ductal adenocarcinomas (PDA) carry mutations in the oncogene KRas which has been proven to be a difficult drug target. P21-activated kinase 4 (PAK4), acts downstream of KRas, and is overexpressed in PDA contributing to its growth and chemoresistance, and thus becomes an attractive therapeutic target. We have developed a new PAK4 inhibitor, PAKib and tested its effect on pancreatic cancer (PC) cell growth in vitro and in a syngeneic mouse model of PC. PAKib suppressed PC cell growth by inducing cell death and cycle arrest. PAKib inhibited PC growth and enhanced the inhibition by gemcitabine of PC in cell culture and in PC mouse model. PAKib acted through multiple signaling pathways involved in cell cycle checkpoints, apoptosis, cell junction, and focal adhesion. These proof-of-concept studies demonstrated the anti-cancer effect of PAKib alone and in combination with gemcitabine and warrant a further clinical investigation.
Keywords: Gemcitabine; KRas; PAK4; Pancreatic cancer.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
MN and JZ are on the Board of Pakinax Pty Ltd that was involved in the manufacture or the PAKib. JZ is current CEO of Pakinax Pty Ltd. HH is a medical advisor to Pakinax Pty Ltd and was involved in the manufacture of PAKib.
Figures




References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. - PubMed
-
- Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, I Australian Pancreatic Cancer Genome. Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. - PMC - PubMed
-
- Bournet B., Buscail C., Muscari F., Cordelier P., Buscail L. Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: Hopes and realities. Eur. J. Cancer. 2016;54:75–83. - PubMed
-
- Yeo D., He H., Baldwin G.S., Nikfarjam M. The role of p21-activated kinases in pancreatic cancer. Pancreas. 2015;44:363–369. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

