Analysis of sodium 2-mercaptoethane sulfonate in rat plasma using high performance liquid chromatography tandem-mass spectrometry
- PMID: 34974317
- PMCID: PMC8792353
- DOI: 10.1016/j.jchromb.2021.123088
Analysis of sodium 2-mercaptoethane sulfonate in rat plasma using high performance liquid chromatography tandem-mass spectrometry
Abstract
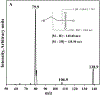
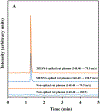
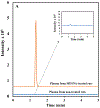
Sodium 2-mercaptoethane sulfonate (MESNA) is a thiol-containing compound that has proven to be effective in inactivating acrolein, the toxic metabolite of some anti-cancer drugs (e.g., cyclophosphamide and ifosphamide). Also, it scavenges free radicals which cause numerous disorders by attacking biological molecules. Current methods available to analyze MESNA in biological matrices include colorimetry and high-performance liquid chromatography (HPLC) with ultraviolet, fluorescence, or electrochemical detection. These methods have several limitations including low sensitivity, poor selectivity, a high degree of difficulty, and long analysis times. Hence, a rapid, simple, and sensitive HPLC tandem mass spectrometry (MS/MS) method was developed and validated to quantify MESNA in rat plasma following IP administration. The analysis of MESNA was accomplished via plasma protein precipitation, centrifugation, supernatant evaporation, reconstitution, and HPLC-MS/MS analysis. The method showcases an outstanding limit of detection (20 nM), excellent linearity (R2 = 0.999, and percent residual accuracy >90%) and a wide linear range (0.05-200 μM). The method also produced good accuracy and precision (100 ± 10% and <10% relative standard deviation, respectively). The validated method was successfully used to analyze MESNA from treated animals and will allow easier development of MESNA for therapeutic purposes.
Keywords: Antineoplastic; Chemoprotectant; Free radical; LC-MS/MS; Method development.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
High performance liquid chromatography analysis of MESNA (2-mercaptoethane sulfonate) in biological samples using fluorescence detection.Biomed Chromatogr. 2005 Jan;19(1):80-6. doi: 10.1002/bmc.420. Biomed Chromatogr. 2005. PMID: 15372507
-
Simultaneous determination of BNP7787 and its metabolite mesna in plasma and tissue by micro-HPLC with a dual electrochemical detector.J Pharm Sci. 2003 May;92(5):1040-50. doi: 10.1002/jps.10363. J Pharm Sci. 2003. PMID: 12712424
-
Simultaneous determination of ten active constituents of Yankening Capsule in rat plasma by ultra high performance liquid chromatography with tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Jan 26;978-979:43-53. doi: 10.1016/j.jchromb.2014.10.016. Epub 2014 Oct 22. J Chromatogr B Analyt Technol Biomed Life Sci. 2015. PMID: 25531869
-
Development and validation of an ultra-high-performance liquid chromatography-tandem mass spectrometry method to determine the bioavailability of warfarin and its major metabolite 7-hydroxy warfarin in rats dosed with oral formulations containing different polymorphic forms.Biomed Chromatogr. 2019 Dec;33(12):e4685. doi: 10.1002/bmc.4685. Epub 2019 Sep 9. Biomed Chromatogr. 2019. PMID: 31430835
-
Determination of 3-mercaptopyruvate in rabbit plasma by high performance liquid chromatography tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Feb 15;949-950:94-8. doi: 10.1016/j.jchromb.2014.01.006. Epub 2014 Jan 18. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 24480329 Free PMC article.
Cited by
-
Mesna Improves Outcomes of Sulfur Mustard Inhalation Toxicity in an Acute Rat Model.J Pharmacol Exp Ther. 2024 Jan 17;388(2):576-585. doi: 10.1124/jpet.123.001683. J Pharmacol Exp Ther. 2024. PMID: 37541763 Free PMC article.
References
-
- Dechant KL, Brogden RN, Pilkington T, Faulds D, Ifosfamide/mesna, Drugs. 42 (1991) 428–467. - PubMed
-
- Shaw IC, Graham MI, Mesna—a short review, Cancer Treatment Reviews. 14 (1987) 67–86. - PubMed
-
- Verschraagen M, Bosma M, Zwiers THU, Torun E, van der Vijgh WJF, Quantification of mesna and total mesna in kidney tissue by high-performance liquid chromatography with electrochemical detection, Journal of Chromatography B. 783 (2003) 33–42. - PubMed
-
- Laauser C, Habekost G, Habekost A, Mass Spectrometric Detection and Reductive Degradation of the Anti-cancer Drugs Ifosfamide and Cyclophosphamide, Applied Ecology and Environmental Sciences. 6 (2018) 15–22. 10.12691/aees-6-1-3. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

