De-escalation of biological therapy in inflammatory bowel disease patients following prior dose escalation
- PMID: 34974465
- PMCID: PMC8983943
- DOI: 10.1097/MEG.0000000000002336
De-escalation of biological therapy in inflammatory bowel disease patients following prior dose escalation
Abstract
Background: Limited data are available on biological therapy de-escalation after prior escalation in inflammatory bowel disease (IBD) patients. This study aimed to assess the frequency and success rate of de-escalation of biological therapy in IBD patients after prior dose escalation and to evaluate which measures are used to guide de-escalation.
Methods: This multicentre retrospective cohort study enrolled IBD patients treated with infliximab (IFX), adalimumab (ADA) or vedolizumab (VEDO) in whom therapy was de-escalated after prior biological escalation. De-escalations were considered pharmacokinetic-driven if based on clinical symptoms combined with therapeutic or supratherapeutic trough levels, and disease activity-driven if based on faecal calprotectin less than or equal to 200 µg/g or resolution of perianal fistula drainage or closure or endoscopic remission. Successful de-escalation was defined as remaining on the same or lower biological dose for greater than or equal to 6 months after de-escalation without the need for corticosteroids.
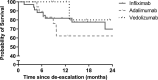
Results: In total, 206 IFX users, 85 ADA users and 55 VEDO users underwent therapy escalation. Of these patients, 34 (17%) on IFX, 18 (21%) on ADA and 8 (15%) on VEDO underwent therapy de-escalation. De-escalation was successful in 88% of IFX patients, 89% of ADA and 100% of VEDO. The probability of remaining on the de-escalated regimen or further de-escalation after 1 year was 85% for IFX, 62% for ADA and 100% for VEDO. Disease activity-driven de-escalations were more often successful (97%) than pharmacokinetic- and no marker-driven de-escalations (76%); P = 0.017.
Conclusion: De-escalation after biological dose escalation was successful in the majority of carefully selected IBD patients. Objective assessment of remission increased the likelihood of successful de-escalation.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
R.L.W. has participated in advisory boards, or as a speaker or consultant for the following companies: Abbvie and Janssen. J.M.J. has served on advisory boards, or as a speaker or consultant for Abbvie, Amgen, Ferring, Fresenius, Janssen, MSD, Pfizer and Takeda. T.E.H.R. has served as a speaker or consultant for Ferring, Janssen and Takeda. F.H. has served on advisory boards, or as a speaker or consultant for Abbvie, Celgene, Janssen Cilag, MSD, Takeda, Celltrion, Teva, Sandoz and Dr Falk, and has received unrestricted grants from Dr Falk, Janssen-Cilag and Abbvie. For the remaining authors, there are no conflicts of interest.
Figures

References
-
- Baert F, Glorieus E, Reenaers C, D’Haens G, Peeters H, Franchimont D, et al. ; BIRD (Belgian IBD Research and Development). Adalimumab dose escalation and dose de-escalation success rate and predictors in a large national cohort of Crohn’s patients. J Crohns Colitis 2013; 7:154–160. - PubMed
-
- Van de Vondel S, Baert F, Reenaers C, Vanden Branden S, Amininejad L, Dewint P, et al. Incidence and predictors of success of adalimumab dose escalation and de-escalation in ulcerative colitis: a real-world Belgian Cohort Study. Inflamm Bowel Dis 2018; 24:1099–1105. - PubMed
-
- Qiu Y, Chen BL, Mao R, Zhang SH, He Y, Zeng ZR, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol 2017; 52:535–554. - PubMed
-
- Peyrin-Biroulet L, Danese S, Argollo M, Pouillon L, Peppas S, Gonzalez-Lorenzo M, et al. Loss of response to vedolizumab and ability of dose intensification to restore response in patients with Crohn’s disease or ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019; 17:838–846.e2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

