Increased expression of pro-inflammatory cytokines at the fetal-maternal interface in bovine pregnancies produced by cloning
- PMID: 34974639
- PMCID: PMC9285385
- DOI: 10.1111/aji.13520
Increased expression of pro-inflammatory cytokines at the fetal-maternal interface in bovine pregnancies produced by cloning
Abstract
Problem: A significant rate of spontaneous abortion is observed in cattle pregnancies produced by somatic cell nuclear transfer (SCNT). Major histocompatibility complex class I (MHC-I) proteins are abnormally expressed on the surface of trophoblast cells from SCNT conceptuses.
Method of study: MHC-I homozygous compatible (n = 9), homozygous incompatible (n = 8), and heterozygous incompatible (n = 5) pregnancies were established by SCNT. Eight control pregnancies were established by artificial insemination. Uterine and trophoblast samples were collected on day 35 ±1 of pregnancy, the expression of immune-related genes was examined by qPCR, and the expression of trophoblast microRNAs was assessed by sequencing.
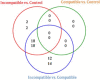
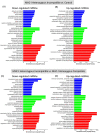
Results: Compared to the control group, trophoblast from MHC-I heterozygous incompatible pregnancies expressed increased levels of CD28, CTLA4, CXCL8, IFNG, IL1A, IL2, IL10, IL12B, TBX21, and TNF, while GNLY expression was downregulated. The MHC-I homozygous incompatible treatment group expressed increased levels of IFNG, IL1A, and IL2 while the MHC-I homozygous compatible group did not differentially express any genes compared to the control group. In the endometrium, relative to the control group, MHC-I heterozygous incompatible pregnancies expressed increased levels of CD28, CTLA4, CXCL8, IFNG, IL10, IL12B, and TNF, while GATA3 expression was downregulated. The MHC-I homozygous incompatible group expressed decreased amounts of CSF2 transcripts compared with the control group but did not have abnormal expression of any other immune-related genes. MHC-I incompatible pregnancies had 40 deregulated miRNAs compared to control pregnancies and 62 deregulated microRNAs compared to MHC-I compatible pregnancies.
Conclusions: MHC-I compatibility between the dam and fetus prevented an exacerbated maternal immune response from being mounted against fetal antigens.
Keywords: cattle; cytokines; gene expression; microRNA; miscarriage; pregnancy; somatic cell nuclear transfer.
© 2022 The Authors. American Journal of Reproductive Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Figures


References
-
- Panarace M, Aguero JI, Garrote M, et al. How healthy are clones and their progeny: 5 years of field experience. Theriogenology. 2007;67(1):142–151. - PubMed
-
- Cibelli JB, Stice SL, Golueke PJ, et al. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science. 1998;280(5367):1256–1258. - PubMed
-
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810–813. - PubMed
-
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full‐term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394(6691):369–374. - PubMed
-
- Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod. 1999;60(4):996–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

