Dexmedetomidine attenuates ischemia and reperfusion-induced cardiomyocyte injury through p53 and forkhead box O3a (FOXO3a)/p53-upregulated modulator of apoptosis (PUMA) signaling signaling
- PMID: 34974801
- PMCID: PMC8805856
- DOI: 10.1080/21655979.2021.2017611
Dexmedetomidine attenuates ischemia and reperfusion-induced cardiomyocyte injury through p53 and forkhead box O3a (FOXO3a)/p53-upregulated modulator of apoptosis (PUMA) signaling signaling
Retraction in
-
Statement of Retraction: Dexmedetomidine attenuates ischemia and reperfusion-induced cardiomyocyte injury through p53 and forkhead box O3a (FOXO3a)/p53-upregulated modulator of apoptosis (PUMA) signaling signaling.Bioengineered. 2024 Dec;15(1):2299625. doi: 10.1080/21655979.2024.2299625. Epub 2024 Feb 20. Bioengineered. 2024. PMID: 38376902 Free PMC article. No abstract available.
Abstract
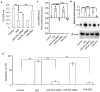
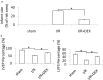
Dexmedetomidine (DEX) has been reported to attenuate the ischemia and reperfusion (I/R) induced cardiomyocyte apoptosis. However, mechanisms underlying these protective effect remain to be fully elucidated. Cardiomyocyte apoptosis is associated with ischemic heart disease. Here we investigated the role of DEX in I/R -induced cardiomyocyte apoptosis. Mice and H9c2 cardiomyocyte cells were subjected to cardiomyocyte I/R injury and hypoxia/reoxygenation (H/R) injury, respectively. The roles and mechanisms of DEX on H9c2 cardiomyocyte cells and mice cardiomyocyte cells exposured to H/R or I/R injury were explored. The results showed that DEX attenuates H/R injury-induced H9c2 cell apoptosis and alleviated mitochondrial oxidative stress; it also reduced myocardial infarct size and protected the cardiac function following cardiomyocyte I/R injury. In addition, H/R and I/R injury increased p53 expression and forkhead box O3a (FOXO3a)/p53-upregulated modulator of apoptosis (PUMA) signaling in H9c2 cardiomyocyte cells and cardiomyocytes. Targeting p53 expression or FOXO3a/PUMA signaling inhibited cell apoptosis and protected against H/R injury in H9c2 cardiomyocyte cells and cardiomyocytes. Pretreatment with DEX reduced the H/R or I/R injury-induced activation of p53 expression and FOXO3a/PUMA signaling, and alleviated H/R or I/R injury-induced apoptosis and mitochondrial oxidative stress. Therefore, DEX could alleviate H/R- or I/R-induced cardiomyocytes injury by reducing cell apoptosis and blocking p53 expression and FOXO3a/PUMA signaling. Targeting p53 or/and FOXO3a/PUMA signaling could alleviate cardiomyocyte I/R injury.
Keywords: Dexmedetomidine; apoptosis; forkhead box o3a; ischemia and reperfusion; p53; p53-upregulated modulator of apoptosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Belleville JP, Ward DS, Bloor BC, et al. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77(6):1125–1133. - PubMed
-
- Wunsch H, Kahn JM, Kramer AA, et al. Dexmedetomidine in the care of critically ill patients from 2001 to 2007: an observational cohort study. Anesthesiology. 2010;113(2):386–394. - PubMed
-
- Xia R, Yin H, Xia ZY, et al. Effect of intravenous infusion of dexmedetomidine combined with inhalation of isoflurane on arterial oxygenation and intrapulmonary shunt during single-lung ventilation. Cell Biochem Biophys. 2013;67(3):1547–1550. - PubMed
-
- Xiang H, Hu B, Li Z, et al. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37(5):1763–1770. - PubMed
-
- Hoffman WE, Kochs E, Werner C, et al. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75(2):328–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
