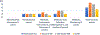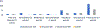Evaluation of Patient-Reported Outcomes (PROs) Protocol Content and Reporting for Clinical Trials that Lead to the approval of frontline Immune Checkpoint Blockade Combination for Patients with Advanced Renal Cell Carcinoma - The Patients' Voice or a Missed Opportunity
- PMID: 34974985
- PMCID: PMC10285347
- DOI: 10.1016/j.clgc.2021.12.002
Evaluation of Patient-Reported Outcomes (PROs) Protocol Content and Reporting for Clinical Trials that Lead to the approval of frontline Immune Checkpoint Blockade Combination for Patients with Advanced Renal Cell Carcinoma - The Patients' Voice or a Missed Opportunity
Abstract
Introduction: Immune checkpoint blockade (ICB) is a rapidly emerging field of oncology that has revolutionized the metastatic renal cell carcinoma (mRCC) treatment. Four recent treatment regimens Nivolumab-Ipilimumab, Pembrolizumab-Axitinib, Nivolumab-Cabozantinib, and Pembrolizumab-Lenvatinib-have demonstrated improved clinical endpoints compared to standard of care and are endorsed by NCCN (2021). However, data on patient-reported outcomes (PROs) for patients receiving these regimens are limited. We conducted a comparative assessment of the quality and standardization of PROs endpoints and data reported for these randomized controlled trials (RCTs).
Patients and methods: We systematically identified all RCTs evaluating combination ICB for ccRCC. PROs-specific data were abstracted from the final version of 4 RCT protocols, as well as clinical and PROs specific manuscripts published between April 2018 and April 2021. We used 3 previously published guides standardizing PROs research to objectively score the data: (i) 24-point PROEAS; (ii) 12-point SPIRIT-PRO; and (iii) 14-point CONSORT-PRO.
Results: The CheckMate 214, KEYNOTE 426, CheckMate 9ER, and CLEAR studies had PROEAS scores of 88% (21/24), 37% (9/24), 83% (20/24), and 16% (4/24), respectively, and SPIRIT-PRO scores of 50% (6/12), 75% (9/12), 66% (8/12), and 41% (5/12) respectively. The CONSORT-PRO scores were 86% (12/14) for CheckMate 214 and 43% (6/14) for CheckMate 9ER, but scores were not available for the CLEAR and KEYNOTE 426 studies because of a lack of sufficient data. The average SPIRIT-PRO score across the 4 RCTs was 58%, indicating a reasonable adoption of PROs research in data management and analysis. The CheckMate 214 trial had the longest follow-up and most comprehensive published PROs data.
Conclusion: Our analysis identified the limitations of current PROs data in combination ICB approved for mRCC. This analysis will enable clinicians to better interpret the current PROs results and emphasize the importance of better incorporation of PROs endpoints in future mRCC trial design.
Keywords: Clear cell carcinoma; Clinical Trial Design; Health related quality of life; Kidney Cancer; Metastatic renal cell carcinoma.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest Disclosure:
HJ has been a consultant for RedHill Biopharma, Janssen Scientific Affairs, Merck and has received funding from Kite Pharma. BM is a NCCN committee member. PS is a NCCN Vice Chair committee member. JAC has been a consultant for Exelixis and Pfizer.
Figures
References
-
- Nivolumab Combined With Ipilimumab Versus Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma (CheckMate 214) - Full Text View - ClinicalTrials.gov. Accessed May 6, 2021. https://clinicaltrials.gov/ct2/show/NCT02231749
-
- Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK-3475) in Combination With Axitinib Versus Sunitinib Monotherapy in Participants With Renal Cell Carcinoma (MK-3475-426/KEYNOTE-426) - Study Results - ClinicalTrials.gov. Accessed May 6, 2021. https://clinicaltrials.gov/ct2/show/results/NCT02853331
-
- A Study of Nivolumab Combined With Cabozantinib Compared to Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma - Full Text View - ClinicalTrials.gov. Accessed May 6, 2021. https://clinicaltrials.gov/ct2/show/NCT03141177
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous