Structural and Functional Aspects of the Neurodevelopmental Gene NR2F1: From Animal Models to Human Pathology
- PMID: 34975398
- PMCID: PMC8715095
- DOI: 10.3389/fnmol.2021.767965
Structural and Functional Aspects of the Neurodevelopmental Gene NR2F1: From Animal Models to Human Pathology
Abstract
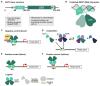
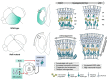
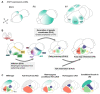
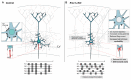
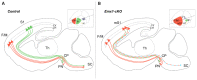
The assembly and maturation of the mammalian brain result from an intricate cascade of highly coordinated developmental events, such as cell proliferation, migration, and differentiation. Any impairment of this delicate multi-factorial process can lead to complex neurodevelopmental diseases, sharing common pathogenic mechanisms and molecular pathways resulting in multiple clinical signs. A recently described monogenic neurodevelopmental syndrome named Bosch-Boonstra-Schaaf Optic Atrophy Syndrome (BBSOAS) is caused by NR2F1 haploinsufficiency. The NR2F1 gene, coding for a transcriptional regulator belonging to the steroid/thyroid hormone receptor superfamily, is known to play key roles in several brain developmental processes, from proliferation and differentiation of neural progenitors to migration and identity acquisition of neocortical neurons. In a clinical context, the disruption of these cellular processes could underlie the pathogenesis of several symptoms affecting BBSOAS patients, such as intellectual disability, visual impairment, epilepsy, and autistic traits. In this review, we will introduce NR2F1 protein structure, molecular functioning, and expression profile in the developing mouse brain. Then, we will focus on Nr2f1 several functions during cortical development, from neocortical area and cell-type specification to maturation of network activity, hippocampal development governing learning behaviors, assembly of the visual system, and finally establishment of cortico-spinal descending tracts regulating motor execution. Whenever possible, we will link experimental findings in animal or cellular models to corresponding features of the human pathology. Finally, we will highlight some of the unresolved questions on the diverse functions played by Nr2f1 during brain development, in order to propose future research directions. All in all, we believe that understanding BBSOAS mechanisms will contribute to further unveiling pathophysiological mechanisms shared by several neurodevelopmental disorders and eventually lead to effective treatments.
Keywords: BBSOAS; NR2F1; cortical development; mouse models; neurodevelopmental disease.
Copyright © 2021 Tocco, Bertacchi and Studer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Adam F., Sourisseau T., Métivier R., Le Page Y., Desbois C., Michel D., et al. . (2000). COUP-TFI (chicken ovalbumin upstream promoter-transcription factor I) regulates cell migration and axogenesis in differentiating P19 embryonal carcinoma cells. Mol. Endocrinol. 14, 1918–1933. 10.1210/mend.14.12.0562 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

