Orphan Medicine Incentives: How to Address the Unmet Needs of Rare Disease Patients by Optimizing the European Orphan Medicinal Product Landscape Guiding Principles and Policy Proposals by the European Expert Group for Orphan Drug Incentives (OD Expert Group)
- PMID: 34975469
- PMCID: PMC8717920
- DOI: 10.3389/fphar.2021.744532
Orphan Medicine Incentives: How to Address the Unmet Needs of Rare Disease Patients by Optimizing the European Orphan Medicinal Product Landscape Guiding Principles and Policy Proposals by the European Expert Group for Orphan Drug Incentives (OD Expert Group)
Abstract
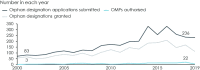
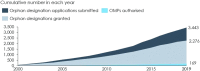
Today policy makers face the challenge to devise a policy framework that improves orphan medicinal product (OMP) development by creating incentives to deliver treatments where there are none and to authorize innovative and transformative treatments where treatments already exist. The European Expert Group on Orphan Drug Incentives (hereafter, OD Expert Group) came together in 2020 to develop policy proposals to facilitate EU policy makers to meet this challenge. The group brings together representatives of the broad rare disease community, including researchers, academia, patient representatives, members of the investor community, rare disease companies and trade associations. The group's work builds on the recognition that only an ambitious policy agenda developed in a multi-stakeholder setting can bring about the quantum leap needed to address unmet needs of rare disease patients today. Along the OMP development path, the OD Expert Group has identified four main needs that a policy revision should address: 1) Need to improve the R&D ecosystem for basic research and company take-up of development. 2) Need to improve the system of financial incentives and rewards. 3) Need to improve the flexibility, predictability and speed of the regulatory pathway. 4) Need to improve the coherence and predictability of demand and pricing for OMPs. This article presents the results of the OD Expert Group work as a set of guiding principles that the revision of the policy framework should follow and a set of 14 policy proposals that address the main needs of OMP development in Europe today.
Keywords: OMP regulation; incentives; orphan drug; orphan medicine; rare disease; unmet need.
Copyright © 2021 Aartsma-Rus, Dooms and Le Cam.
Conflict of interest statement
The authors declare that this study received funding from Alexion, Biogen, Bristol Myers Squibb, Chiesi, PTC Therapeutics, Takeda and the European Confederation of Pharmaceutical Entrepreneurs. The funders provided expertise and unrestricted funding to conduct the research and supported coordination and meetings that resulted in the policy document.
Figures





References
-
- Alacrita (2018). Pharmaceutical Probability of Success 2018. Availableat: https://www.alacrita.com/whitepapers/pharmaceutical-probability-of-success (Accessed April 21, 2021).
-
- Dolon (2020). Estimated Impact of EU Orphan Regulation on Incentives for Innovation. Availableat: https://dolon.com/dolon/wp-content/uploads/2020/10/Estimated-impact-of-E... (Accessed April 21, 2021).
-
- Dooms M., Goodwin G., van der Zanden T. M., de Wildt S. N. (2018). Declaration on Good Off-Label Use Practice. Availableat: https://www.braincouncil.eu/wp-content/uploads/2018/07/GOLUP_Declaration... (Accessed April 20, 2021).
LinkOut - more resources
Full Text Sources

