The Preventive Effect of Decorin on Epidural Fibrosis and Epidural Adhesions After Laminectomy
- PMID: 34975478
- PMCID: PMC8716848
- DOI: 10.3389/fphar.2021.774316
The Preventive Effect of Decorin on Epidural Fibrosis and Epidural Adhesions After Laminectomy
Abstract
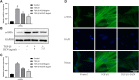
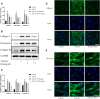
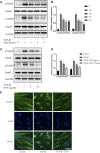
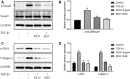
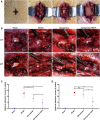
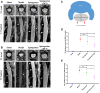
Laminectomy is commonly performed to treat degenerative spinal diseases by reducing compression on the spinal cord and nerve roots. The postoperative epidural fibrosis and epidural adhesions may result in failed back surgery syndrome, which is characterized by the symptoms of lower back pain or leg pain. There is currently no satisfactory treatment for this complication. The pathological processes of epidural fibrosis and epidural adhesions are relevant to the proliferation of fibroblasts, transdifferentiation of fibroblasts into myofibroblasts, and the excessive deposition of extracellular matrix (ECM) protein. According to reports, transforming growth factor-β1 (TGF-β1) played a vital role in the development of fibrosis by promoting aforementioned processes. Decorin, an endogenous proteoglycan and natural inhibitor of TGF-β1, has exhibited prominent anti-fibrosis activity in various scar formation and fibrosis models of many organs. However, the preventive effect of decorin on epidural fibrosis and epidural adhesions requires further investigation. Here, we investigated the therapeutic effects and potential mechanisms of decorin on epidural fibrosis and epidural adhesions. Our results indicated that decorin could significantly suppress the TGF-β1-induced proliferation, transdifferentiation, and extracellular matrix production in primary fibroblasts. Furthermore, Smad2/3 signaling pathway had been demonstrated to be involved in the preventive effect of decorin. Moreover, administration of decorin in vivo could notably inhibit epidural fibrosis and epidural adhesions after laminectomy. To date, there is no approved therapy to target TGF-β1 for the treatment of epidural fibrosis and epidural adhesions after laminectomy. Our research proved the anti-fibrosis effect of decorin, which may provide an effective and promising treatment for epidural fibrosis and epidural adhesions.
Keywords: Smad2/3; decorin; epidural adhesion; epidural fibrosis; fibroblast; laminectomy.
Copyright © 2021 Ding, Wei, Sheng, Wang, Jing, Ma, Zhang, Wang, Li, Tang, Wu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
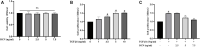



References
LinkOut - more resources
Full Text Sources

