Characterization of Glycosylation-Specific Systemic and Mucosal IgA Antibody Responses to Escherichia coli Mucinase YghJ (SslE)
- PMID: 34975849
- PMCID: PMC8718676
- DOI: 10.3389/fimmu.2021.760135
Characterization of Glycosylation-Specific Systemic and Mucosal IgA Antibody Responses to Escherichia coli Mucinase YghJ (SslE)
Abstract
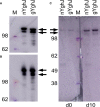
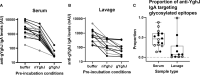
Efforts to develop broadly protective vaccines against pathogenic Escherichia coli are ongoing. A potential antigen candidate for vaccine development is the metalloprotease YghJ, or SslE. YghJ is a conserved mucinase that is immunogenic, heavily glycosylated, and produced by most pathogenic E. coli. To develop efficacious YghJ-based vaccines, there is a need to investigate to what extent potentially protective antibody responses target glycosylated epitopes in YghJ and to describe variations in the quality of YghJ glycosylation in the E. coli population. In this study we estimated the proportion of anti-YghJ IgA antibodies that targeted glycosylated epitopes in serum and intestinal lavage samples from 21 volunteers experimentally infected with wild-type enterotoxigenic E. coli (ETEC) strain TW10722. Glycosylated and non-glycosylated YghJ was expressed, purified, and then gycosylation pattern was verified by BEMAP analysis. Then we used a multiplex bead flow cytometric assay to analyse samples from before and 10 days after TW10722 was ingested. We found that 20 (95%) of the 21 volunteers had IgA antibody responses to homologous, glycosylated YghJ, with a median fold increase in IgA levels of 7.9 (interquartile range [IQR]: 7.1, 11.1) in serum and 3.7 (IQR: 2.1, 10.7) in lavage. The median proportion of anti-YghJ IgA response that specifically targeted glycosylated epitopes was 0.45 (IQR: 0.30, 0.59) in serum and 0.07 (IQR: 0.01, 0.22) in lavage. Our findings suggest that a substantial, but variable, proportion of the IgA antibody response to YghJ in serum during ETEC infection is targeted against glycosylated epitopes, but that gut IgA responses largely target non-glycosylated epitopes. Further research into IgA targeting glycosylated YghJ epitopes is of interest to the vaccine development efforts.
Keywords: IgA; SslE; YghJ; enterotoxigenic Escherichia coli; immunogenicity; protein glycosylation; vaccine development.
Copyright © 2021 Riaz, Steinsland, Thorsing, Andersen, Boysen and Hanevik.
Conflict of interest statement
AB is listed as an inventor on a patent relating to WO 2017/059864 held by the University of Southern Denmark and Aarhus University. AB and AA have a financial interest in GlyProVac ApS, which has licensed exclusively the IP stated above. AB is the scientific founder, shareholder, and a member of the board. AA is a co-founder, shareholder, and a member of the board. AB, AA, and MT are employees of GlyProVac ApS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Moriel DG, Bertoldi I, Spagnuolo A, Marchi S, Rosini R, Nesta B, et al. . Identification of Protective and Broadly Conserved Vaccine Antigens From the Genome of Extraintestinal Pathogenic Escherichia Coli. Proc Natl Acad Sci USA (2010) 107(20):9072–7. doi: 10.1073/pnas.0915077107 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

