The Role of Immune Cells in Post-Stroke Angiogenesis and Neuronal Remodeling: The Known and the Unknown
- PMID: 34975872
- PMCID: PMC8716409
- DOI: 10.3389/fimmu.2021.784098
The Role of Immune Cells in Post-Stroke Angiogenesis and Neuronal Remodeling: The Known and the Unknown
Abstract
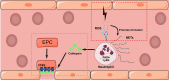
Following a cerebral ischemic event, substantial alterations in both cellular and molecular activities occur due to ischemia-induced cerebral pathology. Mounting evidence indicates that the robust recruitment of immune cells plays a central role in the acute stage of stroke. Infiltrating peripheral immune cells and resident microglia mediate neuronal cell death and blood-brain barrier disruption by releasing inflammation-associated molecules. Nevertheless, profound immunological effects in the context of the subacute and chronic recovery phase of stroke have received little attention. Early attempts to curtail the infiltration of immune cells were effective in mitigating brain injury in experimental stroke studies but failed to exert beneficial effects in clinical trials. Neural tissue damage repair processes include angiogenesis, neurogenesis, and synaptic remodeling, etc. Post-stroke inflammatory cells can adopt divergent phenotypes that influence the aforementioned biological processes in both endothelial and neural stem cells by either alleviating acute inflammatory responses or secreting a variety of growth factors, which are substantially involved in the process of angiogenesis and neurogenesis. To better understand the multiple roles of immune cells in neural tissue repair processes post stroke, we review what is known and unknown regarding the role of immune cells in angiogenesis, neurogenesis, and neuronal remodeling. A comprehensive understanding of these inflammatory mechanisms may help identify potential targets for the development of novel immunoregulatory therapeutic strategies that ameliorate complications and improve functional rehabilitation after stroke.
Keywords: angiogenesis; immune cells; inflammation; ischemic stroke; neurogenesis.
Copyright © 2021 Ma, Yang, He, Zhang and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, et al. . Treatment and Outcome of Hemorrhagic Transformation After Intravenous Alteplase in Acute Ischemic Stroke: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke (2017) 48:e343–61. doi: 10.1161/STR.0000000000000152 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

