Antcin K Inhibits TNF-α, IL-1β and IL-8 Expression in Synovial Fibroblasts and Ameliorates Cartilage Degradation: Implications for the Treatment of Rheumatoid Arthritis
- PMID: 34975889
- PMCID: PMC8714747
- DOI: 10.3389/fimmu.2021.790925
Antcin K Inhibits TNF-α, IL-1β and IL-8 Expression in Synovial Fibroblasts and Ameliorates Cartilage Degradation: Implications for the Treatment of Rheumatoid Arthritis
Abstract
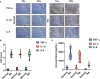
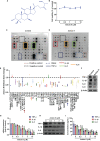
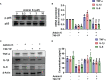
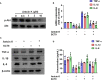
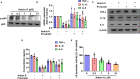
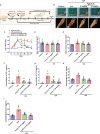
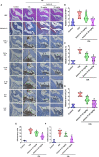
Extracts from Taiwan's traditional medicinal mushroom, Antrodia cinnamomea, exhibit anti-inflammatory activities in cellular and preclinical studies. However, this paper is the first to report that Antcin K, a triterpenoid isolated from A. cinnamomea, inhibits proinflammatory cytokine production in human rheumatoid synovial fibroblasts (RASFs), which are major players in rheumatoid arthritis (RA) disease. In our analysis of the mechanism of action, Antcin K inhibited the expression of three cytokines (tumor necrosis factor alpha [TNF-α], interleukin 1 beta [IL-1β] and IL-8) in human RASFs; cytokines that are crucial to RA synovial inflammation. Notably, incubation of RASFs with Antcin K reduced the phosphorylation of the focal adhesion kinase (FAK), phosphoinositide 3-kinase (PI3K), protein kinase B (AKT) and nuclear factor-κB (NF-κB) signaling cascades, all of which promote cytokine production in RA. Intraperitoneal injections of Antcin K (10 mg/kg or 30 mg/kg) attenuated paw swelling, cartilage degradation and bone erosion, and decreased serum levels of TNF-α, IL-1β, IL-8 in collagen-induced arthritis (CIA) mice; in further experiments, IL-6 levels were similarly reduced. The inhibitory effects of Antcin K upon TNF-α, IL-1β and IL-8 expression in human RASFs was achieved through the downregulation of the FAK, PI3K, AKT and NF-κB signaling cascades. Our data support clinical investigations using Antcin K in RA disease.
Keywords: Antrodia cinnamomea; antcin K; collagen-induced arthritis; interleukin 1 beta; interleukin 8; rheumatoid arthritis; rheumatoid arthritis synovial fibroblast; tumor necrosis factor alpha.
Copyright © 2021 Achudhan, Liu, Lin, Huang, Tsai, Ko, Chiang, Kuo and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

