Anti-SARS-CoV-2 Immunoglobulin Isotypes, and Neutralization Activity Against Viral Variants, According to BNT162b2-Vaccination and Infection History
- PMID: 34975897
- PMCID: PMC8718396
- DOI: 10.3389/fimmu.2021.793191
Anti-SARS-CoV-2 Immunoglobulin Isotypes, and Neutralization Activity Against Viral Variants, According to BNT162b2-Vaccination and Infection History
Abstract
Purpose: To compare SARS-CoV-2 antigen-specific antibody production and plasma neutralizing capacity against B.1 wild-type-like strain, and Gamma/P.1 and Delta/B.1.617.2 variants-of-concern, in subjects with different Covid-19 disease and vaccination histories.
Methods: Adult subjects were: 1) Unvaccinated/hospitalized for Covid-19; 2) Covid-19-recovered followed by one BNT162b2 vaccine dose; and 3) Covid-19-naïve/2-dose BNT162b2 vaccinated. Multiplex Luminex® immunoassays measured IgG, IgA, and IgM plasma levels against SARS-CoV-2 receptor-binding domain (RBD), spike-1 (S), and nucleocapsid proteins. Neutralizing activity was determined in Vero E6 cytopathic assays.
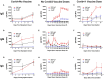
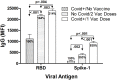
Results: Maximum anti-RBD IgG levels were similar in Covid-19‑recovered individuals 8‒10 days after single-dose vaccination and in Covid-19-naïve subjects 7 days after 2nd vaccine dosing; both groups had ≈2‑fold higher anti-RBD IgG levels than Unvaccinated/Covid-19 subjects tracked through 2 weeks post-symptom onset. Anti-S IgG expression patterns were similar to RBD within each group, but with lower signal strengths. Viral antigen-specific IgA and IgM levels were more variable than IgG patterns. Anti-nucleocapsid immunoglobulins were not detected in Covid-19-naïve subjects. Neutralizing activity against the B.1 strain, and Gamma/P.1 and Delta/B.1.617.2 variants, was highest in Covid‑19-recovered/single-dose vaccinated subjects; although neutralization against the Delta variant in this group was only 26% compared to B.1 neutralization, absolute anti-Delta titers suggested maintained protection. Neutralizing titers against the Gamma and Delta variants were 33‒77% and 26‒67%, respectively, versus neutralization against the B.1 strain (100%) in the three groups.
Conclusion: These findings support SARS-CoV-2 mRNA vaccine usefulness regardless of Covid-19 history, and confirm remarkable protection provided by a single vaccine dose in people who have recovered from Covid-19.
Keywords: COVID-19; SARS-CoV-2; bead; coronavirus; fluorescence; multiplex immunoassay; neutralization assay; serological assay.
Copyright © 2021 Tarkowski, de Jager, Schiuma, Covizzi, Lai, Gabrieli, Corbellino, Bergna, Ventura, Galli, Riva and Antinori.
Conflict of interest statement
WD was an employee of Luminex Corporation during the study period. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Johns Hopkins University, Center for Science and Systems Engineering. Coronavirus Resource Center (2021). Available at: https://coronavirus.jhu.edu/map.html (Accessed October 11, 2021).
-
- Piccoli L, Park YJ, Tortorici MA, Czudnochowski N, Walls AC, Beltramello M, et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell (2020) 183(4):1024‒42.e21. doi: 10.1016/j.cell.2020.09.037 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

