The Distinct Roles of Sialyltransferases in Cancer Biology and Onco-Immunology
- PMID: 34975914
- PMCID: PMC8718907
- DOI: 10.3389/fimmu.2021.799861
The Distinct Roles of Sialyltransferases in Cancer Biology and Onco-Immunology
Abstract
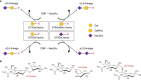
Aberrant glycosylation is a key feature of malignant transformation. Hypersialylation, the enhanced expression of sialic acid-terminated glycoconjugates on the cell surface, has been linked to immune evasion and metastatic spread, eventually by interaction with sialoglycan-binding lectins, including Siglecs and selectins. The biosynthesis of tumor-associated sialoglycans involves sialyltransferases, which are differentially expressed in cancer cells. In this review article, we provide an overview of the twenty human sialyltransferases and their roles in cancer biology and immunity. A better understanding of the individual contribution of select sialyltransferases to the tumor sialome may lead to more personalized strategies for the treatment of cancer.
Keywords: cancer; sialic acid; sialyltransferases; tumor glycosylation; tumor immunology.
Copyright © 2021 Hugonnet, Singh, Haas and von Gunten.
Conflict of interest statement
SVG received remuneration for serving on the scientific advisory board of Palleon Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Oh-Uti K. Polysaccharides and a Glycidamin in the Tissue of Gastric Cancer. Tohoku J Exp Med (1949) 51:297–304. doi: 10.1620/tjem.51.297 - DOI

