Glucose biosensors in clinical practice: principles, limits and perspectives of currently used devices
- PMID: 34976197
- PMCID: PMC8692922
- DOI: 10.7150/thno.64035
Glucose biosensors in clinical practice: principles, limits and perspectives of currently used devices
Abstract
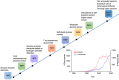
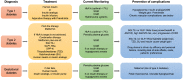
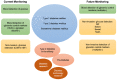
The demand of glucose monitoring devices and even of updated guidelines for the management of diabetic patients is dramatically increasing due to the progressive rise in the prevalence of diabetes mellitus and the need to prevent its complications. Even though the introduction of the first glucose sensor occurred decades ago, important advances both from the technological and clinical point of view have contributed to a substantial improvement in quality healthcare. This review aims to bring together purely technological and clinical aspects of interest in the field of glucose devices by proposing a roadmap in glucose monitoring and management of patients with diabetes. Also, it prospects other biological fluids to be examined as further options in diabetes care, and suggests, throughout the technology innovation process, future directions to improve the follow-up, treatment, and clinical outcomes of patients.
Keywords: assessment of glycemic control; biological fluids; diabetes technology; glucose sensors; point-of-care testing..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Battelino T, Bergenstal RM. Continuous glucose monitoring-derived data report-simply a better management tool. Diabetes Care. 2020;43:2327–9. - PubMed
-
- Caruso R, Rebora P, Dellafiore F, Fabrizi D, Riegel B, Ausili D. et al. Clinical and socio-demographic determinants of inadequate self-care in adults with type 1 diabetes mellitus: the leading role of self-care confidence. Acta Diabetol. 2019;56:151–61. - PubMed
-
- Adeel M, Rahman MM, Caligiuri I, Canzonieri V, Rizzolio F, Daniele S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens Bioelectron. 2020;165:112331. - PubMed
-
- Islam MS. Diabetes: from research to clinical practice. Adv Exp Med Biol. 2021;1307:1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

