Resveratrol ameliorates polycystic ovary syndrome via transzonal projections within oocyte-granulosa cell communication
- PMID: 34976213
- PMCID: PMC8692920
- DOI: 10.7150/thno.67167
Resveratrol ameliorates polycystic ovary syndrome via transzonal projections within oocyte-granulosa cell communication
Abstract
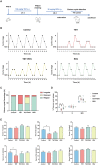
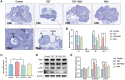
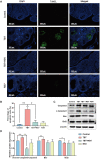
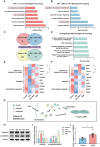
Rationale: Polycystic ovary syndrome (PCOS) is closely linked to follicular dysplasia and impaired bidirectional oocyte-granulosa cell (GC) communication. Given that PCOS is a heterogeneous, multifactorial endocrine disorder, it is important to clarify the pathophysiology of this ovarian disease and identify a specific treatment. Methods: We generated PCOS rat models based on neonatal tributyltin (TBT) exposure and studied the therapeutic effect and mechanism of resveratrol (RSV), a natural plant polyphenol. Transcriptome analysis was conducted to screen the significantly changed pathways, and a series of experiments, such as quantitative real-time polymerase chain reaction (PCR), Western blot and phalloidin staining, were performed in rat ovaries. We also observed similar changes in human PCOS samples using Gene Expression Omnibus (GEO) database analysis and quantitative real-time PCR. Results: We first found that injury to transzonal projections (TZPs), which are specialized filopodia that mediate oocyte-GC communication in follicles, may play an important role in the etiology of PCOS. We successfully established PCOS rat models using TBT and found that overexpressed calcium-/calmodulin-dependent protein kinase II beta (CaMKIIβ) inhibited TZP assembly. In addition, TZP disruption and CAMK2B upregulation were also observed in samples from PCOS patients. Moreover, we demonstrated that RSV potently ameliorated ovarian failure and estrus cycle disorder through TZP recovery via increased cytoplasmic calcium levels and excessive phosphorylation of CaMKIIβ. Conclusions: Our data indicated that upregulation of CaMKIIβ may play a critical role in regulating TZP assembly and may be involved in the pathogenesis of PCOS associated with ovarian dysfunction. Investigation of TZPs and RSV as potent CaMKIIβ activators provides new insight and a therapeutic target for PCOS, which is helpful for improving female reproduction.
Keywords: CaMKIIβ; PCOS; TZP assembly; oocyte-GC communication; resveratrol.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. - PubMed
-
- Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106:6–15. - PubMed
-
- Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

