Safety and Efficacy of VP-102 (Cantharidin, 0.7% w/v) in Molluscum Contagiosum by Body Region: Post hoc Pooled Analyses from Two Phase III Randomized Trials
- PMID: 34976290
- PMCID: PMC8711618
Safety and Efficacy of VP-102 (Cantharidin, 0.7% w/v) in Molluscum Contagiosum by Body Region: Post hoc Pooled Analyses from Two Phase III Randomized Trials
Abstract
Trial registration: >ClinicalTrials.gov identifier nos. NCT03377790 (for CAMP-1) and NCT03377803 (for CAMP-2).
Background: VP-102 is drug-device combination product containing cantharidin (0.7% w/v) and has undergone Phase III clinical trials for the treatment of molluscum contagiosum (molluscum). Efficacy and safety may differ by body region due to variable skin anatomy.
Objective: We investigated the pooled safety and efficacy of VP-102 by affected body region.
Methods: Individuals at least two years of age with molluscum were randomized to topical VP-102 or vehicle once every 21 days until clear (maximum of four applications). Participants were assigned to body region groups where lesions were present at baseline. Body region lesion counts were recorded at each visit. Efficacy was measured by the percentage of participants with complete clearance of lesions by region. Pre-specified adverse events (AEs) were analyzed for those treated in the region on that visit.
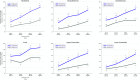
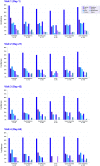
Results: Participants had a mean of two regions affected at baseline. Complete clearance was significantly higher in the VP-102-treated group than with vehicle application in all regions at the last visit (P<0.01 for each region). The incidence of pre-specified AEs was consistent across regions. However, these analyses were post hoc, and individual lesions were not tracked for efficacy.
Conclusion: VP-102 treatment shows consistent safety and efficacy across molluscum body regions.
Keywords: Child; VP-102; cantharidin; incidence; infectious disease; lesion; molluscum; molluscum contagiosum; pediatric; sensitive skin; topical.
Copyright © 2021. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
DISCLOSURES: Dr. Eichenfield received funding to his institution for the described research, has received consulting fees for and stock from Verrica Pharmaceuticals for activities unrelated to this publication, and is a member of the Board of Directors of Verrica Pharmaceuticals. Dr. Kwong has received consulting fees and research funding from Verrica Pharmaceuticals. Dr. Gonzalez has received research funding for the described research and consulting fees from Verrica Pharmaceuticals unrelated to this publication. Dr. Yan has received consulting fees from Verrica Pharmaceuticals for activities unrelated to this publication. Mr. D’Arnaud was a contractor for Verrica Pharmaceuticals. Dr. Burnett was employed by Verrica Pharmaceuticals and holds company stock. Dr. Olivadoti is an employee of Verrica Pharmaceuticals
Figures

References
-
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13(10):877–888. - PubMed
-
- Leung AKC. The natural history of molluscum contagiosum in children. Lancet Infect Dis. 2015;15(2):136–137. - PubMed
-
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Dermatol Rep. 2020;9(1):83–92.
-
- Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130–136. - PubMed
-
- Badri T, Gandhi GR. Molluscum Contagiosum. In: StatPearls. Treasure Island Florida: StatPearls Publishing LLC; 2020:6. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
