Dendrimer-2PMPA selectively blocks upregulated microglial GCPII activity and improves cognition in a mouse model of multiple sclerosis
- PMID: 34976589
- PMCID: PMC8671953
- DOI: 10.7150/ntno.63158
Dendrimer-2PMPA selectively blocks upregulated microglial GCPII activity and improves cognition in a mouse model of multiple sclerosis
Abstract
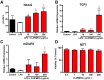
Cognitive impairment is a common aspect of multiple sclerosis (MS) for which there are no treatments. Reduced brain N-acetylaspartylglutamate (NAAG) levels are linked to impaired cognition in various neurological diseases, including MS. NAAG levels are regulated by glutamate carboxypeptidase II (GCPII), which hydrolyzes the neuropeptide to N-acetyl-aspartate and glutamate. GCPII activity is upregulated multifold in microglia following neuroinflammation. Although several GCPII inhibitors, such as 2-PMPA, elevate brain NAAG levels and restore cognitive function in preclinical studies when given at high systemic doses or via direct brain injection, none are clinically available due to poor bioavailability and limited brain penetration. Hydroxyl-dendrimers have been successfully used to selectively deliver drugs to activated glia. Methods: We attached 2-PMPA to hydroxyl polyamidoamine (PAMAM) dendrimers (D-2PMPA) using a click chemistry approach. Cy5-labelled-D-2PMPA was used to visualize selective glial uptake in vitro and in vivo. D-2PMPA was evaluated for anti-inflammatory effects in LPS-treated glial cultures. In experimental autoimmune encephalomyelitis (EAE)-immunized mice, D-2PMPA was dosed biweekly starting at disease onset and cognition was assessed using the Barnes maze, and GCPII activity was measured in CD11b+ hippocampal cells. Results: D-2PMPA showed preferential uptake into microglia and robust anti-inflammatory activity, including elevations in NAAG, TGFβ, and mGluR3 in glial cultures. D-2PMPA significantly improved cognition in EAE mice, even though physical severity was unaffected. GCPII activity increased >20-fold in CD11b+ cells from EAE mice, which was significantly mitigated by D-2PMPA treatment. Conclusions: Hydroxyl dendrimers facilitate targeted drug delivery to activated microglia. These data support further development of D-2PMPA to attenuate elevated microglial GCPII activity and treat cognitive impairment in MS.
Keywords: GCPII; NAAG; cognitive impairment; dendrimer; multiple sclerosis.
© The author(s).
Conflict of interest statement
Competing Interests: Under license agreements involving Ashvattha Therapeutics, LLC and its subsidiary, Orpheris, Inc., and the Johns Hopkins University, Drs. Slusher, Kannan, and Rangaramanujam and the University are entitled to royalty distributions related to technology involved in the study discussed in this publication. Drs. Slusher (Board Member), Kannan (Co-founder, Board Member), and Rangaramanujam (Co-founder, Board Member) hold equity in Ashvattha Therapeutics Inc. Additionally, the study discussed in this publication was funded by and involved a dendrimer-drug manufactured by Ashvattha Therapeutics, LLC. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. AS, RS and SPK are co-inventors of patents licensed by Ashvattha, relating to the dendrimer platform. All other authors declare that there are no conflicts of interest.
Figures






References
-
- Benedict RH, Wahlig E, Bakshi R, Fishman I, Munschauer F, Zivadinov R. et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231:29–34. - PubMed
-
- Campbell J, Rashid W, Cercignani M, Langdon D. Cognitive impairment among patients with multiple sclerosis: associations with employment and quality of life. Postgrad Med J. 2017;93:143–7. - PubMed
-
- Vornov JJ, Hollinger KR, Jackson PF, Wozniak KM, Farah MH, Majer P. et al. Still NAAG'ing After All These Years: The Continuing Pursuit of GCPII Inhibitors. Adv Pharmacol. 2016;76:215–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

