Exploring the translational potential of clusterin as a biomarker of early osteoarthritis
- PMID: 34976733
- PMCID: PMC8671091
- DOI: 10.1016/j.jot.2021.10.001
Exploring the translational potential of clusterin as a biomarker of early osteoarthritis
Abstract
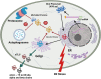
Background: Clusterin (CLU; also known as apolipoprotein J) is an ATP-independent holdase chaperone that prevents proteotoxicity as a consequence of protein aggregation. It is a ∼60 kDa disulfide-linked heterodimeric protein involved in the clearance of cellular debris and the regulation of apoptosis. CLU has been proposed to protect cells from cytolysis by complement components and has been implicated in Alzheimer's disease due to its ability to bind amyloid-β peptides and prevent aggregate formation in the brain. Recent studies suggest that CLU performs moonlighting functions. CLU exists in two major forms: an intracellular form and a secreted extracellular form. The intracellular form of CLU may suppress stress-induced apoptosis by forming complexes with misfolded proteins and facilitates their degradation. The secreted form of CLU functions as an extracellular chaperone that prevents protein aggregation.
Methods: In this review, we discuss the published literature on the biology of CLU in cartilage, chondrocytes, and other synovial joint tissues. We also review clinical studies that have examined the potential for using this protein as a biomarker in synovial and systemic fluids of patients with rheumatoid arthritis (RA) or osteoarthritis (OA).
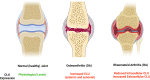
Results: Since CLU functions as an extracellular chaperone, we propose that it may be involved in cytoprotective functions in osteoarticular tissues. The secreted form of CLU can be measured in synovial and systemic fluids and may have translational potential as a biomarker of early repair responses in OA.
Conclusion: There is significant potential for investigating synovial and systemic CLU as biomarkers of OA. Future translational and clinical orthopaedic studies should carefully consider the diverse roles of this protein and its involvement in other comorbidities. Therefore, future biomarker studies should not correlate circulating CLU levels exclusively to the process of OA pathogenesis and progression. Special attention should be paid to CLU levels in synovial fluid.
The translational potential of this article: There is significant potential for investigating synovial and systemic CLU as a predictive biomarker of osteoarthritis (OA) progression and response to novel treatments and interventions. Given that CLU plays diverse roles in other comorbidities such as rheumatoid arthritis (RA) and obesity, future translational and clinical orthopaedic biomarker studies should not directly correlate circulating CLU levels to the process of OA pathogenesis and progression. However, special attention should be paid to CLU levels in synovial fluid. The cytoprotective properties of CLU may support the implementation of regenerative strategies and new approaches for developing targeted therapeutics for OA.
Keywords: ACL, anterior cruciate ligament; ACR, American College of Rheumatology; ApoJ, apolipoprotein J; Apoptosis; CLU, clusterin; CMC-I, carpometacarpal joint; COMP, cartilage oligomeric matrix protein; Clusterin (CLU); ECM, extracellular matrix; ELISA, enzyme-linked immunosorbent assay; ESCEO, The European Society for Clinical and Economic Aspects of Osteoporosis: Osteoarthritis and Musculoskeletal Diseases; Inflammation; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International; Osteoarthritis (OA); PsA, psoriatic arthritis; RA, rheumatoid arthritis; Rheumatoid arthritis (RA); SF, synovial fluid; TNF-α, tumor necrosis factor-α; Translational biomarker; hsCRP, high sensitivity C-reactive protein; qRT-PCR, quantitative reverse transcription polymerase chain reaction; sCLU, secreted clusterin.
© 2021 Published by Elsevier (Singapore) Pte Ltd on behalf of Chinese Speaking Orthopaedic Society.
Conflict of interest statement
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures
References
-
- Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014 Jul;73(7):1323–1330. - PubMed
-
- Hunter D.J., March L., Chew M. Osteoarthritis in 2020 and beyond: a lancet commission. Lancet. 2020 Nov 28;396:1711–1712. 10264. - PubMed
-
- Felson D.T., Naimark A., Anderson J., Kazis L., Castelli W., Meenan R.F. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987 Aug;30(8):914–918. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

