High-fat western diet-consumption alters crystalline silica-induced serum adipokines, inflammatory cytokines and arterial blood flow in the F344 rat
- PMID: 34976743
- PMCID: PMC8683385
- DOI: 10.1016/j.toxrep.2021.12.001
High-fat western diet-consumption alters crystalline silica-induced serum adipokines, inflammatory cytokines and arterial blood flow in the F344 rat
Abstract
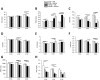
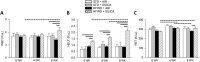
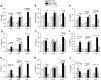
Adipose tissue (AT) plays a central role in the maintenance of whole-body energy homeostasis through release of adipokines. High-fat Western diet (HFWD)-consumption contributes to obesity, disruption of adipocyte metabolism, chronic systemic inflammation, and metabolic dysfunction (MetDys). MetDys is associated with impaired lung function, pulmonary hypertension, and asthma. Thirty-five percent of adults in the U.S. have MetDys, yet the impact of MetDys on susceptibility to occupational hazards is unknown. The aim of this study was to determine the potential of HFWD-consumption to alter inhaled crystalline silica dust-induced metabolic responses. Six-wk old male F344 rats were fed a HFWD (45 kcal % fat, sucrose 22.2 % by weight) or standard rat chow (STD, controls), and exposed to silica-inhalation (6 h/d, 5 d/wk, 39 d; Min-U-Sil 5®, 15 mg/m3) or filtered air. Indices of MetDys and systemic inflammation were measured at 0, 4, and 8 wk following cessation of silica exposure. At 8 wk post-exposure, silica reduced serum leptin and adiponectin levels, and increased arterial pulse frequency. HFWD-consumption induced weight gain, altered adipokines, liver, kidney, and pancreatic function, and increased tail artery blood flow. At 8 wk in HFWD + SIL-treated animals, the levels of serum pro-inflammatory cytokines (IFN-γ, CXCL-1, TNF-α, IL-1β, IL-4, IL-5, IL-6, IL-10 and IL-13) were increased compared to STD + SIL but were less than HFWD + AIR-induced levels. In conclusion, consumption of a HFWD altered silica-induced metabolic responses and silica exposure disrupted AT endocrine function. These findings demonstrate previously unknown interactions between HFWD-consumption and occupational silica exposure.
Keywords: Adipokines; Adipose tissue; Cytokines; Inflammation; Obesity; Silicosis.
© 2021 The Authors.
Conflict of interest statement
The authors report no declarations of interest.
Figures

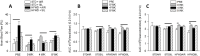
References
LinkOut - more resources
Full Text Sources
Research Materials

