Development and Validation of an Immune-Related Signature for the Prediction of Recurrence Risk of Patients With Laryngeal Cancer
- PMID: 34976784
- PMCID: PMC8716380
- DOI: 10.3389/fonc.2021.683915
Development and Validation of an Immune-Related Signature for the Prediction of Recurrence Risk of Patients With Laryngeal Cancer
Abstract
Objective: Our purpose was to develop and verify an immune-related signature for predicting recurrence risk of patients with laryngeal cancer.
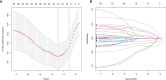
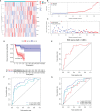
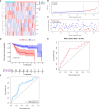
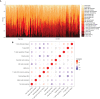
Methods: RNA-seq data of 51 recurrence and 81 non-recurrence laryngeal cancer samples were downloaded from TCGA database, as the training set. Microarray data of 34 recurrence and 75 non-recurrence cancer samples were obtained from GEO dataset, as the validation set. Single factor cox regression was utilized to screen prognosis-related immune genes. After LASSO regression analysis, an immune-related signature was constructed. Recurrence free survival (RFS) between high- and low- recurrence risk patients was presented, followed by ROC. We also evaluated the correlation between immune infiltration and the signature using the CIBERSORT algorithm. The genes in the signature were validated in laryngeal cancer tissues by western blot or RT-qPCR. After RCN1 knockdown, migration and invasion of laryngeal cancer cells were investigated.
Results: Totally, 43 prognosis-related immune genes were identified for laryngeal cancer. Among them, eight genes were used for constructing a prognostic signature. High risk group exhibited a higher recurrence risk than low risk group. The AUC for 1-year was separately 0.803 and 0.715 in the training and verification sets, suggesting its well efficacy for predicting the recurrence. Furthermore, this signature was closely related to distinct immune cell infiltration. RCN1, DNAJA2, LASP1 and IBSP were up-regulated in laryngeal cancer. RCN1 knockdown restrained migrated and invasive abilities of laryngeal cancer cells.
Conclusion: Our findings identify a reliable immune-related signature that can predict the recurrence risk of patients with laryngeal cancer.
Keywords: immune; laryngeal cancer; prognosis; recurrence; signature.
Copyright © 2021 Zhang, Zhao, Wang and Ji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Zhu X, Heng Y, Zhou L, Tao L, Zhang M. A Prognostic Nomogram for Predicting Risk of Recurrence in Laryngeal Squamous Cell Carcinoma Patients After Tumor Resection to Assist Decision Making for Postoperative Adjuvant Treatment. J Surg Oncol (2019) 120(4):698–706. doi: 10.1002/jso.25614 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

