EGFR-Dependent Extracellular Matrix Protein Interactions Might Light a Candle in Cell Behavior of Non-Small Cell Lung Cancer
- PMID: 34976811
- PMCID: PMC8714827
- DOI: 10.3389/fonc.2021.766659
EGFR-Dependent Extracellular Matrix Protein Interactions Might Light a Candle in Cell Behavior of Non-Small Cell Lung Cancer
Abstract
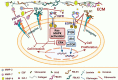
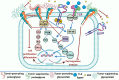
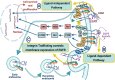
Lung cancer remains the leading cause of cancer-related death and is associated with a poor prognosis. Lung cancer is divided into 2 main types: the major in incidence is non-small cell lung cancer (NSCLC) and the minor is small cell lung cancer (SCLC). Although NSCLC progression depends on driver mutations, it is also affected by the extracellular matrix (ECM) interactions that activate their corresponding signaling molecules in concert with integrins and matrix metalloproteinases (MMPs). These signaling molecules include cytoplasmic kinases, small GTPases, adapter proteins, and receptor tyrosine kinases (RTKs), particularly the epidermal growth factor receptor (EGFR). In NSCLC, the interplay between ECM and EGFR regulates ECM stiffness, angiogenesis, survival, adhesion, migration, and metastasis. Furthermore, some tumor-promoting ECM components (e.g., glycoproteins and proteoglycans) enhance activation of EGFR and loss of PTEN. On the other hand, other tumor-suppressing glycoproteins and -proteoglycans can inhibit EGFR activation, suppressing cell invasion and migration. Therefore, deciphering the molecular mechanisms underlying EGFR and ECM interactions might provide a better understanding of disease pathobiology and aid in developing therapeutic strategies. This review critically discusses the crosstalk between EGFR and ECM affecting cell behavior of NSCLC, as well as the involvement of ECM components in developing resistance to EGFR inhibition.
Keywords: epidermal growth factor receptor (EGFR); extracellular matrix (ECM); glycoproteins; integrin receptors; matrix metalloproteinases (MMPs); non-small cell lung cancer (NSCLC); proteoglycans; tyrosine kinase inhibitors (TKIs)..
Copyright © 2021 Hassanein, Abdel-Mawgood and Ibrahim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cell Derived Matrix Fibulin-1 Associates With Epidermal Growth Factor Receptor to Inhibit Its Activation, Localization and Function in Lung Cancer Calu-1 Cells.Front Cell Dev Biol. 2020 Jul 3;8:522. doi: 10.3389/fcell.2020.00522. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32719793 Free PMC article.
-
Tumor immune microenvironment in epidermal growth factor receptor-mutated non-small cell lung cancer before and after epidermal growth factor receptor tyrosine kinase inhibitor treatment: a narrative review.Transl Lung Cancer Res. 2021 Sep;10(9):3823-3839. doi: 10.21037/tlcr-21-572. Transl Lung Cancer Res. 2021. PMID: 34733631 Free PMC article. Review.
-
Epidermal Growth Factor Receptor Mutation (EGFR) Testing for Prediction of Response to EGFR-Targeting Tyrosine Kinase Inhibitor (TKI) Drugs in Patients with Advanced Non-Small-Cell Lung Cancer: An Evidence-Based Analysis.Ont Health Technol Assess Ser. 2010;10(24):1-48. Epub 2010 Dec 1. Ont Health Technol Assess Ser. 2010. PMID: 23074402 Free PMC article.
-
Targeting the MET gene for the treatment of non-small-cell lung cancer.Crit Rev Oncol Hematol. 2014 Feb;89(2):284-99. doi: 10.1016/j.critrevonc.2013.11.006. Epub 2013 Dec 1. Crit Rev Oncol Hematol. 2014. PMID: 24355409 Review.
-
Mutational activation of the epidermal growth factor receptor down-regulates major histocompatibility complex class I expression via the extracellular signal-regulated kinase in non-small cell lung cancer.Cancer Sci. 2019 Jan;110(1):52-60. doi: 10.1111/cas.13860. Epub 2018 Nov 27. Cancer Sci. 2019. PMID: 30390416 Free PMC article.
Cited by
-
Different Tyrosine Kinase Inhibitors Used in Treating EGFR-Mutant Pulmonary Adenocarcinoma with Brain Metastasis and Intracranial Intervention Have No Impact on Clinical Outcomes.Cancers (Basel). 2022 Dec 28;15(1):187. doi: 10.3390/cancers15010187. Cancers (Basel). 2022. PMID: 36612183 Free PMC article.
-
CDKL3 Promotes Non-small Cell Lung Cancer by Suppressing Autophagy Via Activation of PI3K/Akt/mTOR Pathway.Mol Biotechnol. 2023 Sep;65(9):1421-1431. doi: 10.1007/s12033-023-00656-8. Epub 2023 Jan 11. Mol Biotechnol. 2023. PMID: 36630073
-
Oncogenic and immunological role of EDIL3 in human tumours: From pan-cancer analysis to validation in gastric cancer.Heliyon. 2024 Jun 1;10(11):e32291. doi: 10.1016/j.heliyon.2024.e32291. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38882287 Free PMC article.
-
Competing Subclones and Fitness Diversity Shape Tumor Evolution Across Cancer Types.bioRxiv [Preprint]. 2025 Jun 3:2025.05.31.657191. doi: 10.1101/2025.05.31.657191. bioRxiv. 2025. PMID: 40502073 Free PMC article. Preprint.
-
TRANS: a prediction model for EGFR mutation status in NSCLC based on radiomics and clinical features.Respir Res. 2025 Jun 5;26(1):211. doi: 10.1186/s12931-025-03287-6. Respir Res. 2025. PMID: 40474176 Free PMC article.
References
-
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. . The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

