Prognostic Factors and Clinical Characteristics of Duodenal Adenocarcinoma With Survival: A Retrospective Study
- PMID: 34976838
- PMCID: PMC8715708
- DOI: 10.3389/fonc.2021.795891
Prognostic Factors and Clinical Characteristics of Duodenal Adenocarcinoma With Survival: A Retrospective Study
Abstract
Background: To evaluate the clinical risk factors that influence the overall survival in patients with duodenal adenocarcinoma (DA) after tumor resection.
Methods: This study retrospectively analyzed 188 patients who underwent tumor resection for DA between January 2005 and June 2020 at Xiangyang Central Hospital.
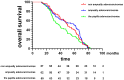
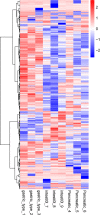
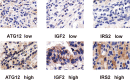
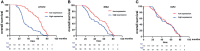
Results: The median survival of the patients who underwent resectional operation was 54 months, longer than of those who underwent palliative surgery (20.8 months) (2,916.17; 95% CI, 916.3-9,280.5; p < 0.001). Survival of non-ampullary duodenal carcinoma patients (50.3 months; 95% CI, 39.7-61.8) was similar to that of ampullary duodenal carcinoma patients (59.3 months; 95% CI, 38.6-66.7) but was significantly better than that of papillary adenocarcinoma patients (38.9 months; 95% CI, 29.8-54.8; p = 0.386). Those with intestinal-type ductal adenocarcinomas had a longer median overall survival than those with the gastric type (61.8 vs. 46.7 months; p < 0.01) or pancreatic type (32.2 months; p < 0.001). Clinical DA samples had significantly diverse expressions of ATG12, IRS2, and IGF2. Higher expressions of the ATG12 and IRS2 proteins were significantly correlated with worse survival. Multivariate Cox regression analysis revealed that lymph node metastasis (hazard ratio (HR), 6.44; 95% CI, 3.68-11.27; p < 0.0001), margin status (HR, 4.94; 95% CI, 2.85-8.54; p < 0.0001), and high expression of ATG12 (HR, 1.89; 95% CI, 1.17-3.06; p = 0.0099) were independent prognostic factors negatively associated with survival in patients undergoing curative resection. There was no survival difference between the groups with ampullary, non-ampullary, and papillary adenocarcinomas treated with adjuvant chemotherapy (p = 0.973).
Conclusion: Gastric/pancreatic type, high expression of ATG12, lymph node metastases, and margin status were negative prognosticators of survival in patients with DAs than in those with tumor anatomical location. Curative resection is the best treatment option for appropriate patients.
Keywords: ATG12; duodenal adenocarcinoma; histopathological phenotype; lymph node metastases; overall survival.
Copyright © 2021 Sun, Liu, Lv, Li, Liao and Gong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Prognostic Factors and Clinical Characteristics of Patients with Primary Duodenal Adenocarcinoma: A Single-Center Experience from China.Biomed Res Int. 2016;2016:6491049. doi: 10.1155/2016/6491049. Epub 2016 Dec 27. Biomed Res Int. 2016. PMID: 28116301 Free PMC article.
-
Association of Histopathologic Phenotype of Periampullary Adenocarcinomas With Survival.JAMA Surg. 2017 Jan 1;152(1):82-88. doi: 10.1001/jamasurg.2016.3466. JAMA Surg. 2017. PMID: 27732711
-
Intestinal-type of differentiation predicts favourable overall survival: confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin.BMC Cancer. 2013 Sep 22;13:428. doi: 10.1186/1471-2407-13-428. BMC Cancer. 2013. PMID: 24053229 Free PMC article.
-
Prognostic relevance of the posterior resection margin for predicting disease free survival in ampullary adenocarcinoma.Surg Oncol. 2020 Dec;35:211-217. doi: 10.1016/j.suronc.2020.08.028. Epub 2020 Aug 26. Surg Oncol. 2020. PMID: 32911213
-
Outcomes and Treatment Options for Duodenal Adenocarcinoma: A Systematic Review and Meta-Analysis.Ann Surg Oncol. 2018 Sep;25(9):2681-2692. doi: 10.1245/s10434-018-6567-6. Epub 2018 Jun 26. Ann Surg Oncol. 2018. PMID: 29946997 Free PMC article.
Cited by
-
Rare Case of Duodenal Metastasis From Colon Cancer: Review of Literature and Insights on Novel Therapies.Case Rep Gastrointest Med. 2025 Jul 22;2025:8864636. doi: 10.1155/crgm/8864636. eCollection 2025. Case Rep Gastrointest Med. 2025. PMID: 40740271 Free PMC article.
-
Development and validation of lymph node ratio-based nomograms for primary duodenal adenocarcinoma after surgery.Front Oncol. 2022 Oct 4;12:962381. doi: 10.3389/fonc.2022.962381. eCollection 2022. Front Oncol. 2022. PMID: 36276093 Free PMC article.
-
Multicenter, single-arm, phase II study (CAP) of radiotherapy plus liposomal irinotecan followed by camrelizumab and anti-angiogenic treatment in advanced solid tumors.Front Immunol. 2023 Mar 28;14:1133689. doi: 10.3389/fimmu.2023.1133689. eCollection 2023. Front Immunol. 2023. PMID: 37056765 Free PMC article. Clinical Trial.
-
Clinical Outcome of Resected Non-Ampullary Duodenal Adenocarcinoma: A Single Center Experience.J Clin Med. 2022 Dec 27;12(1):210. doi: 10.3390/jcm12010210. J Clin Med. 2022. PMID: 36615011 Free PMC article.
-
Segmental Duodenal Resections: Toward Defining Indications, Complexity, and Coding.J Gastrointest Surg. 2023 Nov;27(11):2373-2379. doi: 10.1007/s11605-023-05837-z. Epub 2023 Sep 25. J Gastrointest Surg. 2023. PMID: 37749459
References
LinkOut - more resources
Full Text Sources
Miscellaneous

