Vitreoscilla filiformis Extract for Topical Skin Care: A Review
- PMID: 34976852
- PMCID: PMC8717924
- DOI: 10.3389/fcimb.2021.747663
Vitreoscilla filiformis Extract for Topical Skin Care: A Review
Abstract
The term probiotic has been defined by experts as live microorganisms, which when administered in adequate amounts, confer a health benefit on the host. Probiotics are, thus, by definition, live microorganisms, and the viability of probiotics is a prerequisite for certain benefits, such as the release of metabolites at the site or adhesion properties, for example. However, some semi-active or non-replicative bacterial preparations may retain a similar activity to the live forms. On cosmetic, lysates or fractions are generally used. Topically applied Vitreoscilla filiformis extract has shown to have some similar biological activity of probiotics in the gut, for example, regulating immunity by optimisation of regulatory cell function, protecting against infection, and helping skin barrier function for better recovery and resistance. Due to their mode of action and efficacy, V. filiformis extract (lysate including membrane and cytosol) may be considered as non-replicative probiotic fractions, and this review article presents all its properties.
Keywords: Vitreoscilla; immunity; microbiome; skin barrier; skin defences.
Copyright © 2021 Gueniche, Liboutet, Cheilian, Fagot, Juchaux and Breton.
Conflict of interest statement
All authors are employees of L’Oréal Research & Innovation, France.
Figures

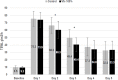
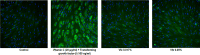
References
-
- Ameen A., Abdulridha M., Najeeb A. (2019). Comparative Effectiveness of Probiotics Timing Regimen in Helicobacter Pylori-Induced Peptic Ulcer Disease Patients. J. Pharm. Sci. & Res 11, 75–83.
-
- Benno Y., He F., Hosoda M., Hashimoto H., Kojima T., Yamazaki K., et al. (1996). Effects of Lactobacillus GG Yogurt on Human Intestinal Microecology in Japanese Subjects. Nutr. Today 31, 12S. doi: 10.1097/00017285-199611001-00004 - DOI
-
- Bergey D. H., Holt J. G. (1994). 9th eds. Bergey's Manual of Determinative Bacteriology (Baltimore: The Williams and Wilkins Co; ), p. 837–53.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

