The Microbiome as a Key Regulator of Female Genital Tract Barrier Function
- PMID: 34976864
- PMCID: PMC8719631
- DOI: 10.3389/fcimb.2021.790627
The Microbiome as a Key Regulator of Female Genital Tract Barrier Function
Abstract
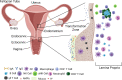
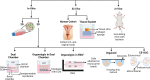
The microbiome, the collection of microbial species at a site or compartment, has been an underappreciated realm of human health up until the last decade. Mounting evidence suggests the microbiome has a critical role in regulating the female genital tract (FGT) mucosa's function as a barrier against sexually transmitted infections (STIs) and pathogens. In this review, we provide the most recent experimental systems and studies for analyzing the interplay between the microbiome and host cells and soluble factors with an influence on barrier function. Key components, such as microbial diversity, soluble factors secreted by host and microbe, as well as host immune system, all contribute to both the physical and immunologic aspects of the FGT mucosal barrier. Current gaps in what is known about the effects of the microbiome on FGT mucosal barrier function are compared and contrasted with the literature of the gut and respiratory mucosa. This review article presents evidence supporting that the vaginal microbiome, directly and indirectly, contributes to how well the FGT protects against infection.
Keywords: barrier; female genital tract (FGT); host factors; microbial factors; microbiome; sexually transmitted infection (STI); tissue explant; vagina.
Copyright © 2021 Plesniarski, Siddik and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aldunate M., Srbinovski D., Hearps A. C., Latham C. F., Ramsland P. A., Gugasyan R., et al. . (2015). Antimicrobial and Immune Modulatory Effects of Lactic Acid and Short Chain Fatty Acids Produced by Vaginal Microbiota Associated With Eubiosis and Bacterial Vaginosis. Front. Physiol. 6, 164. doi: 10.3389/FPHYS.2015.00164/BIBTEX - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

