Salix spp. Bark Hot Water Extracts Show Antiviral, Antibacterial, and Antioxidant Activities-The Bioactive Properties of 16 Clones
- PMID: 34976988
- PMCID: PMC8716786
- DOI: 10.3389/fbioe.2021.797939
Salix spp. Bark Hot Water Extracts Show Antiviral, Antibacterial, and Antioxidant Activities-The Bioactive Properties of 16 Clones
Abstract
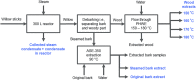
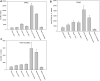
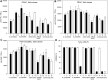
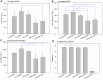
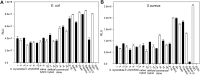
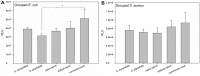
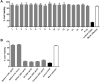
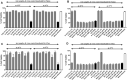
Earlier studies have shown that the bark of Salix L. species (Salicaceae family) is rich in extractives, such as diverse bioactive phenolic compounds. However, we lack knowledge on the bioactive properties of the bark of willow species and clones adapted to the harsh climate conditions of the cool temperate zone. Therefore, the present study aimed to obtain information on the functional profiles of northern willow clones for the use of value-added bioactive solutions. Of the 16 willow clones studied here, 12 were examples of widely distributed native Finnish willow species, including dark-leaved willow (S. myrsinifolia Salisb.) and tea-leaved willow (S. phylicifolia L.) (3 + 4 clones, respectively) and their natural and artificial hybrids (3 + 2 clones, respectively). The four remaining clones were commercial willow varieties from the Swedish willow breeding program. Hot water extraction of bark under mild conditions was carried out. Bioactivity assays were used to screen antiviral, antibacterial, antifungal, yeasticidal, and antioxidant activities, as well as the total phenolic content of the extracts. Additionally, we introduce a fast and less labor-intensive steam-debarking method for Salix spp. feedstocks. Clonal variation was observed in the antioxidant properties of the bark extracts of the 16 Salix spp. clones. High antiviral activity against a non-enveloped enterovirus, coxsackievirus A9, was found, with no marked differences in efficacy between the native clones. All the clones also showed antibacterial activity against Staphylococcus aureus and Escherichia coli, whereas no antifungal (Aspergillus brasiliensis) or yeasticidal (Candida albicans) efficacy was detected. When grouping the clone extract results into Salix myrsinifolia, Salix phylicifolia, native hybrid, artificial hybrid, and commercial clones, there was a significant difference in the activities between S. phylicifolia clone extracts and commercial clone extracts in the favor of S. phylicifolia in the antibacterial and antioxidant tests. In some antioxidant tests, S. phylicifolia clone extracts were also significantly more active than artificial clone extracts. Additionally, S. myrsinifolia clone extracts showed significantly higher activities in some antioxidant tests than commercial clone extracts and artificial clone extracts. Nevertheless, the bark extracts of native Finnish willow clones showed high bioactivity. The obtained knowledge paves the way towards developing high value-added biochemicals and other functional solutions based on willow biorefinery approaches.
Keywords: Salix spp.; antimicrobial; antioxidant; antiviral; bark; debarking; water-extracts.
Copyright © 2021 Tienaho, Reshamwala, Sarjala, Kilpeläinen, Liimatainen, Dou, Viherä-Aarnio, Linnakoski, Marjomäki and Jyske.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Willow (Salix spp.) bark hot water extracts inhibit both enveloped and non-enveloped viruses: study on its anti-coronavirus and anti-enterovirus activities.Front Microbiol. 2023 Nov 8;14:1249794. doi: 10.3389/fmicb.2023.1249794. eCollection 2023. Front Microbiol. 2023. PMID: 38029113 Free PMC article.
-
Polyphenols and Phenolic Glucosides in Antibacterial Twig Extracts of Naturally Occurring Salix myrsinifolia (Salisb.), S. phylicifolia (L.) and S. starkeana (Willd.) and the Cultivated Hybrid S. x pendulina (Wender.).Pharmaceutics. 2024 Jul 9;16(7):916. doi: 10.3390/pharmaceutics16070916. Pharmaceutics. 2024. PMID: 39065613 Free PMC article.
-
Effect of Hybrid Type and Harvesting Season on Phytochemistry and Antibacterial Activity of Extracted Metabolites from Salix Bark.J Agric Food Chem. 2022 Mar 9;70(9):2948-2956. doi: 10.1021/acs.jafc.1c08161. Epub 2022 Feb 24. J Agric Food Chem. 2022. PMID: 35200036 Free PMC article.
-
Efficacy and Safety of White Willow Bark (Salix alba) Extracts.Phytother Res. 2015 Aug;29(8):1112-6. doi: 10.1002/ptr.5377. Epub 2015 May 22. Phytother Res. 2015. PMID: 25997859 Review.
-
Heat Stress in Broiler Chickens and the Effect of Dietary Polyphenols, with Special Reference to Willow (Salix spp.) Bark Supplements-A Review.Antioxidants (Basel). 2021 Apr 27;10(5):686. doi: 10.3390/antiox10050686. Antioxidants (Basel). 2021. PMID: 33925609 Free PMC article. Review.
Cited by
-
Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts.Molecules. 2022 Apr 28;27(9):2817. doi: 10.3390/molecules27092817. Molecules. 2022. PMID: 35566174 Free PMC article.
-
Antibacterial and Oxidative Stress-Protective Effects of Five Monoterpenes from Softwood.Molecules. 2022 Jun 17;27(12):3891. doi: 10.3390/molecules27123891. Molecules. 2022. PMID: 35745011 Free PMC article.
-
Effect of meta-Topolin on morphological, physiochemical, and molecular dynamics during in vitro regeneration of Salix tetrasperma Roxb.BMC Plant Biol. 2025 Jan 28;25(1):121. doi: 10.1186/s12870-025-06095-8. BMC Plant Biol. 2025. PMID: 39875827 Free PMC article.
-
Inspired by nature: Fiber networks functionalized with tannic acid and condensed tannin-rich extracts of Norway spruce bark show antimicrobial efficacy.Front Bioeng Biotechnol. 2023 Apr 19;11:1171908. doi: 10.3389/fbioe.2023.1171908. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37152647 Free PMC article.
-
Selected strains of the Ganoderma lucidum complex from Finnish forests have excellent broadly acting antiviral properties.Sci Rep. 2025 Jul 2;15(1):23565. doi: 10.1038/s41598-025-08377-5. Sci Rep. 2025. PMID: 40604067 Free PMC article.
References
-
- Bobleter O. (1994). Hydrothermal Degradation of Polymers Derived from Plants. Prog. Polym. Sci. 19, 797–841. 10.1016/0079-6700(94)90033-7 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

