Midkine Prevents Calcification of Aortic Valve Interstitial Cells via Intercellular Crosstalk
- PMID: 34977035
- PMCID: PMC8714929
- DOI: 10.3389/fcell.2021.794058
Midkine Prevents Calcification of Aortic Valve Interstitial Cells via Intercellular Crosstalk
Abstract
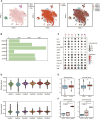
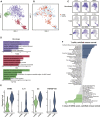
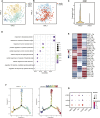
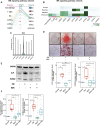
Calcified aortic valve disease (CAVD), the most common valvular heart disease, lacks pharmaceutical treatment options because its pathogenesis remains unclear. This disease with a complex macroenvironment characterizes notable cellular heterogeneity. Therefore, a comprehensive understanding of cellular diversity and cell-to-cell communication are essential for elucidating the mechanisms driving CAVD progression and developing therapeutic targets. In this study, we used single-cell RNA sequencing (scRNA-seq) analysis to describe the comprehensive transcriptomic landscape and cell-to-cell interactions. The transitional valvular endothelial cells (tVECs), an intermediate state during the endothelial-to-mesenchymal transition (EndMT), could be a target to interfere with EndMT progression. Moreover, matrix valvular interstitial cells (mVICs) with high expression of midkine (MDK) interact with activated valvular interstitial cells (aVICs) and compliment-activated valvular interstitial cells (cVICs) through the MK pathway. Then, MDK inhibited calcification of VICs that calcification was validated by Alizarin Red S staining, real-time quantitative polymerase chain reaction (RT-qPCR), and Western blotting assays in vitro. Therefore, we speculated that mVICs secreted MDK to prevent VICs' calcification. Together, these findings delineate the aortic valve cells' heterogeneity, underlining the importance of intercellular cross talk and MDK, which may offer a potential therapeutic strategy as a novel inhibitor of CAVD.
Keywords: CAVD; VICs’ calcification; cell communication; midkine (MDK); scRNA-seq.
Copyright © 2021 Zhou, Cao, Hang, Liang, Zhu, Fan, Shi, Dong and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Valvular interstitial cells suppress calcification of valvular endothelial cells.Atherosclerosis. 2015 Sep;242(1):251-260. doi: 10.1016/j.atherosclerosis.2015.07.008. Epub 2015 Jul 17. Atherosclerosis. 2015. PMID: 26232165 Free PMC article.
-
Human and porcine aortic valve endothelial and interstitial cell isolation and characterization.Front Cardiovasc Med. 2023 Jun 20;10:1151028. doi: 10.3389/fcvm.2023.1151028. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37408661 Free PMC article.
-
MicroRNA-34c Inhibits Osteogenic Differentiation and Valvular Interstitial Cell Calcification via STC1-Mediated JNK Pathway in Calcific Aortic Valve Disease.Front Physiol. 2020 Aug 31;11:829. doi: 10.3389/fphys.2020.00829. eCollection 2020. Front Physiol. 2020. Retraction in: Front Physiol. 2024 Dec 16;15:1540235. doi: 10.3389/fphys.2024.1540235. PMID: 32982764 Free PMC article. Retracted.
-
Endothelial-to-Mesenchymal Transition in Calcific Aortic Valve Disease.Acta Cardiol Sin. 2020 May;36(3):183-194. doi: 10.6515/ACS.202005_36(3).20200213A. Acta Cardiol Sin. 2020. PMID: 32425433 Free PMC article. Review.
-
Development of calcific aortic valve disease: Do we know enough for new clinical trials?J Mol Cell Cardiol. 2019 Jul;132:189-209. doi: 10.1016/j.yjmcc.2019.05.016. Epub 2019 May 25. J Mol Cell Cardiol. 2019. PMID: 31136747 Review.
Cited by
-
Shared gene characteristics and molecular mechanisms of macrophages M1 polarization in calcified aortic valve disease.Front Cardiovasc Med. 2023 Jan 4;9:1058274. doi: 10.3389/fcvm.2022.1058274. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36684607 Free PMC article.
-
Midkine-A novel player in cardiovascular diseases.Front Cardiovasc Med. 2022 Sep 20;9:1003104. doi: 10.3389/fcvm.2022.1003104. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36204583 Free PMC article. Review.
-
Identifying multicellular spatiotemporal organization of cells with SpaceFlow.Nat Commun. 2022 Jul 14;13(1):4076. doi: 10.1038/s41467-022-31739-w. Nat Commun. 2022. PMID: 35835774 Free PMC article.
-
Focusing on the Native Matrix Proteins in Calcific Aortic Valve Stenosis.JACC Basic Transl Sci. 2023 Mar 29;8(8):1028-1039. doi: 10.1016/j.jacbts.2023.01.009. eCollection 2023 Aug. JACC Basic Transl Sci. 2023. PMID: 37719438 Free PMC article. Review.
-
Recent Advances in Deciphering Normal and Diseased Aortic Valve Biology Using Transcriptomic Technologies.J Cell Mol Med. 2025 Sep;29(17):e70835. doi: 10.1111/jcmm.70835. J Cell Mol Med. 2025. PMID: 40903834 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

