Trends of Phase I Clinical Trials of New Drugs in Mainland China Over the Past 10 Years (2011-2020)
- PMID: 34977078
- PMCID: PMC8718673
- DOI: 10.3389/fmed.2021.777698
Trends of Phase I Clinical Trials of New Drugs in Mainland China Over the Past 10 Years (2011-2020)
Abstract
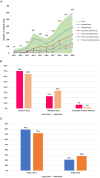
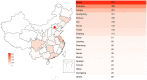
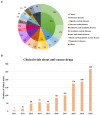
Background: In recent years, the number of clinical trials initiated in China has increased rapidly. The aim of this study was to overview the changing landscape of phase I clinical trials in mainland China from 2011 to 2020. Methods: We analyzed phase I clinical trials registered on 3 websites including the Chinese Clinical Trial Registry, ClinicalTrials.gov, and the China National Medical Products Administration Center for Drug Evaluation platform. Findings: A total of 2,842 phase I clinical trials were posted from January 1, 2011, to December 31, 2020. The overall number of clinical trials for innovative drugs was 1,497, accounting for half of all the phase I clinical trials (53%). Among these 1,486 innovative drug clinical trials, 924 were newly tested drugs with an average annual growth rate of 59%. Biological drug research increased significantly from 22.6% during 2011-2015 to 33.3% during 2016-2020. These principal investigators (PIs) of these clinical trials were mainly from Beijing (n = 871), followed by Shanghai (n = 496) and Jiangsu (n = 281). As for the therapeutic area of phase I clinical trials, cancer took up the most percentage of all the clinical trials (35%), followed by infectious disease (9%), nervous system disease (9%), etc. Most phase I clinical trials are conducted on healthy volunteers (n = 1,642, 57.8%), some cancer drugs are conducted in patients with cancer (n = 846, 29.8%), and only a few clinical trials were conducted in the elderly (n = 7). Among these clinical trials of the newly tested innovative drugs, the first in human (FIH) clinical trials accounted for 82% (744), and the First in Chinese (FIC) clinical trials only took up 18% (167). Only a small number of drugs could be made the transition to phase II (n = 207, 22%). In addition, despite the number of newly tested drugs during 2011-2015 (n = 163) was much less than that in 2016-2020 (n = 761), the percentage of drugs that could enter into phase II clinical trials in 2011-2015 (34%) was higher than that in 2016-2020 (20%). Conclusion: In the past 10 years, the development of phase I clinical trials has achieved great progress in mainland China due to the novel design and drug innovation policy. Nevertheless, future efforts are needed to make for improving the phase transition success rate of innovative drugs.
Keywords: geographical distribution; innovative drugs; phase I clinical trial; phase transition; therapeutic area.
Copyright © 2021 Chen, Lou, Zheng, Wang, Chen and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The changing landscape of anti-lung cancer drug clinical trials in mainland China from 2005 to 2020.Lancet Reg Health West Pac. 2021 Apr 27;11:100151. doi: 10.1016/j.lanwpc.2021.100151. eCollection 2021 Jun. Lancet Reg Health West Pac. 2021. PMID: 34327360 Free PMC article.
-
The changing landscape of anti-lymphoma drug clinical trials in mainland China in the past 15 years (2005-2020): A systematic review.Lancet Reg Health West Pac. 2021 Feb 5;8:100097. doi: 10.1016/j.lanwpc.2021.100097. eCollection 2021 Mar. Lancet Reg Health West Pac. 2021. PMID: 34327425 Free PMC article.
-
Evolution of ischemic stroke drug clinical trials in mainland China from 2005 to 2021.CNS Neurosci Ther. 2022 Aug;28(8):1229-1239. doi: 10.1111/cns.13867. Epub 2022 Jun 1. CNS Neurosci Ther. 2022. PMID: 35642775 Free PMC article.
-
Changes in Clinical Trials of Dermatological Drugs in Mainland China Between 2016 and 2022: A Narrative Review.Ther Innov Regul Sci. 2025 May;59(3):450-461. doi: 10.1007/s43441-025-00743-9. Epub 2025 Feb 13. Ther Innov Regul Sci. 2025. PMID: 39948234 Review.
-
Changes in clinical trials of cancer drugs in mainland China over the decade 2009-18: a systematic review.Lancet Oncol. 2019 Nov;20(11):e619-e626. doi: 10.1016/S1470-2045(19)30491-7. Lancet Oncol. 2019. PMID: 31674320
Cited by
-
Phase I pharmacokinetic, safety, and preliminary efficacy study of tiragolumab in combination with atezolizumab in Chinese patients with advanced solid tumors.Cancer Chemother Pharmacol. 2024 Jul;94(1):45-55. doi: 10.1007/s00280-024-04650-y. Epub 2024 Mar 7. Cancer Chemother Pharmacol. 2024. PMID: 38451273 Free PMC article. Clinical Trial.
-
Analysis of job satisfaction among clinical research coordinators.Work. 2024;79(3):1121-1132. doi: 10.3233/WOR-230732. Work. 2024. PMID: 38875070 Free PMC article.
-
Landscape of the clinical development of China innovative anti-lung cancer drugs.Cancer Pathog Ther. 2022 Oct 11;1(1):67-75. doi: 10.1016/j.cpt.2022.10.003. eCollection 2023 Jan. Cancer Pathog Ther. 2022. PMID: 38328605 Free PMC article. Review.
-
Changes in Early-Phase Clinical Trials in China During 2013-2022: A Review.Drugs R D. 2024 Sep;24(3):383-390. doi: 10.1007/s40268-024-00489-z. Epub 2024 Sep 12. Drugs R D. 2024. PMID: 39266814 Free PMC article. Review.
-
Eligibility criteria in clinical trials in breast cancer: a cohort study.BMC Med. 2023 Jul 3;21(1):240. doi: 10.1186/s12916-023-02947-y. BMC Med. 2023. PMID: 37400830 Free PMC article.
References
-
- Chinese Clinical Trial Registry (ChiCTR) . Available online at: https://www.chictr.org.cn/enindex.aspx (accessed September 10, 2021).
-
- U.S. National Library of Medicine, National Institutes of Health . ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/ (accessed September 10, 2021).
-
- Platform for Registry and Publicity of Drug Clinical Trials . Available online at: http://www.chinadrugtrials.org.cn/index.html (accessed September 10, 2021).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

