DOCK8-expressing T follicular helper cells newly generated beyond self-organized criticality cause systemic lupus erythematosus
- PMID: 34977502
- PMCID: PMC8689056
- DOI: 10.1016/j.isci.2021.103537
DOCK8-expressing T follicular helper cells newly generated beyond self-organized criticality cause systemic lupus erythematosus
Abstract
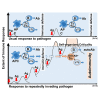
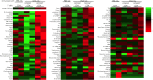
Pathogens including autoantigens all failed to induce systemic lupus erythematosus (SLE). We, instead, studied the integrity of host's immune response that recognized pathogen. By stimulating TCR with an antigen repeatedly to levels that surpass host's steady-state response, self-organized criticality, SLE was induced in mice normally not prone to autoimmunity, wherein T follicular helper (Tfh) cells expressing the guanine nucleotide exchange factor DOCK8 on the cell surface were newly generated. DOCK8+Tfh cells passed through TCR re-revision and induced varieties of autoantibody and lupus lesions. They existed in splenic red pulp and peripheral blood of active lupus patients, which subsequently declined after therapy. Autoantibodies and disease were healed by anti-DOCK8 antibody in the mice including SLE-model (NZBxNZW) F1 mice. Thus, DOCK8+Tfh cells generated after repeated TCR stimulation by immunogenic form of pathogen, either exogenous or endogenous, in combination with HLA to levels that surpass system's self-organized criticality, cause SLE.
Keywords: Cell biology; Immune response; Immunology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Akiyama C., Tsumiyama K., Uchimura C., Honda E., Miyazaki Y., Sakurai K., Miura Y., Hashiramoto A., Shiozawa S. Conditional upregulation of IFN-α alone is sufficient to induce systemic lupus erythematosus. J. Immunol. 2019;203:835–843. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

