Early hepatocellular carcinoma detection using magnetic resonance imaging is cost-effective in high-risk patients with cirrhosis
- PMID: 34977518
- PMCID: PMC8683591
- DOI: 10.1016/j.jhepr.2021.100390
Early hepatocellular carcinoma detection using magnetic resonance imaging is cost-effective in high-risk patients with cirrhosis
Abstract
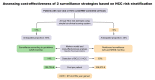
Background & aims: Reinforced hepatocellular carcinoma (HCC) surveillance using magnetic resonance imaging (MRI) could increase early tumour detection but faces cost-effectiveness issues. In this study, we aimed to evaluate the cost-effectiveness of MRI for the detection of very early HCC (Barcelona Clinic Liver Cancer [BCLC] 0) in patients with an annual HCC risk >3%.
Methods: French patients with compensated cirrhosis included in 4 multicentre prospective cohorts were considered. A scoring system was constructed to identify patients with an annual risk >3%. Using a Markov model, the economic evaluation estimated the costs and life years (LYs) gained with MRI vs. ultrasound (US) monitoring over a 20-year period. The incremental cost-effectiveness ratio (ICER) was calculated by dividing the incremental costs by the incremental LYs.
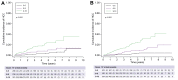
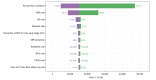
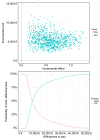
Results: Among 2,513 patients with non-viral causes of cirrhosis (n = 840) and/or cured HCV (n = 1,489)/controlled HBV infection (n = 184), 206 cases of HCC were detected after a 37-month follow-up. When applied to training (n = 1,658) and validation (n = 855) sets, the construction of a scoring system identified 33.4% and 37.5% of patients with an annual HCC risk >3% (3-year C-Indexes 75 and 76, respectively). In patients with a 3% annual risk, the incremental LY gained with MRI was 0.4 for an additional cost of €6,134, resulting in an ICER of €15,447 per LY. Compared to US monitoring, MRI detected 5x more BCLC 0 HCC. The deterministic sensitivity analysis confirmed the impact of HCC incidence. At a willingness to pay of €50,000/LY, MRI screening had a 100% probability of being cost-effective.
Conclusions: In the era of HCV eradication/HBV control, patients with annual HCC risk >3% represent one-third of French patients with cirrhosis. MRI is cost-effective in this population and could favour early HCC detection.
Lay summary: The early identification of hepatocellular carcinoma in patients with cirrhosis is important to improve patient outcomes. Magnetic resonance imaging could increase early tumour detection but is more expensive and less accessible than ultrasound (the standard modality for surveillance). Herein, using a simple score, we identified a subgroup of patients with cirrhosis (accounting for >one-third), who were at increased risk of hepatocellular carcinoma and for whom the increased expense of magnetic resonance imaging would be justified by the potential improvement in outcomes.
Keywords: AFP, alpha-fetoprotein; AMRI, abbreviated magnetic resonance imaging; BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma; HR, hazard ratio; ICER, incremental cost-effectiveness ratio; LY, life years; LYG, life years gained; MRI; MRI, magnetic resonance imaging; NAFLD, non-alcoholic fatty liver disease; QALY, quality-adjusted life year; RFA, radiofrequency ablation; SHR, subdistribution hazard ratio; TACE, transarterial chemoembolization; US, ultrasound; cirrhosis; cost-effectiveness; liver cancer risk; surveillance.
© 2021 The Author(s).
Conflict of interest statement
Pr Nahon has received honoraria from and/or consults for AstraZeneca, Abbvie, Bayer, Bristol-Myers Squibb, Eisai, Gilead, Ipsen, MSD and Roche. He received research grants from AstraZeneca, AbbVie, Bristol-Myers Squibb and Eisai. Pr Ganne-Carrié consults for and/or received personal fees from Abbvie, Bayer, Gilead, Ipsen, and Shionogi, outside the submitted work. Pr Pol has received grants from Gilead, Roche, Abbvie. He consults for BMS, Janssen Cilag, Gilead, Roche, Merck/Schering-Plough, Abbvie, Vivv, Shinogui, Biotest, LFB. Pr Durand-Zaleski consults for AbbVie, Bristol-Myers Squibb, Janssen and MSD. All other authors report no conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- EASL Clinical Practice Guidelines Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. - PubMed
-
- Cadier B., Bulsei J., Nahon P., Seror O., Laurent A., Rosa I., et al. Early detection and curative treatment of hepatocellular carcinoma: a cost-effectiveness analysis in France and in the United States. Hepatology. 2017;65:1237–1248. - PubMed
-
- Costentin C.E., Layese R., Bourcier V., Cagnot C., Marcellin P., Guyader D., et al. Compliance with hepatocellular carcinoma surveillance guidelines associated with increased lead-time adjusted survival of patients with compensated viral cirrhosis: a multi-center cohort study. Gastroenterology. 2018;155:431–442 e410. - PubMed
-
- Trinchet J.C., Chaffaut C., Bourcier V., Degos F., Henrion J., Fontaine H., et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–1997. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

