Design, synthesis and evaluation of tryptophan analogues as tool compounds to study IDO1 activity
- PMID: 34977580
- PMCID: PMC8637876
- DOI: 10.1039/d0cb00209g
Design, synthesis and evaluation of tryptophan analogues as tool compounds to study IDO1 activity
Abstract
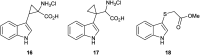
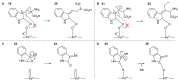
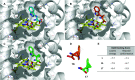
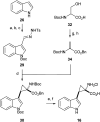
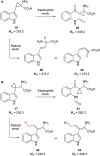
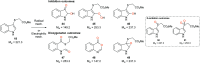
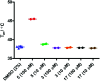
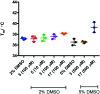
The metabolism of l-tryptophan to N-formyl-l-kynurenine by indoleamine-2,3-dioxygenase 1 (IDO1) is thought to play a critical role in tumour-mediated immune suppression. Whilst there has been significant progress in elucidating the overall enzymatic mechanism of IDO1 and related enzymes, key aspects of the catalytic cycle remain poorly understood. Here we report the design, synthesis and biological evaluation of a series of tryptophan analogues which have the potential to intercept putative intermediates in the metabolism of 1 by IDO1. Functionally-relevant binding to IDO1 was demonstrated through enzymatic inhibition, however no IDO1-mediated metabolism of these compounds was observed. Subsequent T m-shift analysis shows the most active compound, 17, exhibits a distinct profile from known competitive IDO1 inhibitors, with docking studies supporting the hypothesis that 17 may bind at the recently-discovered Si site. These findings provide a start-point for development of further mechanistic probes and more potent tryptophan-based IDO1 inhibitors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
NJC was a PhD student sponsored by Celentyx Ltd. TT, OQ, CAB are employees of Celentyx Ltd with share options in the company. JG and NMB are Directors of Celentyx Ltd with shareholdings in the company. There are no other conflicts to declare.
Figures










Similar articles
-
Discovery and biological evaluation of tanshinone derivatives as potent dual inhibitors of indoleamine 2, 3-dioxygenase 1 and tryptophan 2, 3-dioxygenase.Eur J Med Chem. 2022 May 5;235:114294. doi: 10.1016/j.ejmech.2022.114294. Epub 2022 Mar 16. Eur J Med Chem. 2022. PMID: 35358772
-
Evaluation of Novel Inhibitors of Tryptophan Dioxygenases for Enzyme and Species Selectivity Using Engineered Tumour Cell Lines Expressing Either Murine or Human IDO1 or TDO2.Pharmaceuticals (Basel). 2022 Aug 31;15(9):1090. doi: 10.3390/ph15091090. Pharmaceuticals (Basel). 2022. PMID: 36145311 Free PMC article.
-
Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO.Front Immunol. 2021 Feb 23;12:636081. doi: 10.3389/fimmu.2021.636081. eCollection 2021. Front Immunol. 2021. PMID: 33708223 Free PMC article. Review.
-
Imidazo[2,1-b]thiazole based indoleamine-2,3-dioxygenase 1 (IDO1) inhibitor: Structure based design, synthesis, bio-evaluation and docking studies.Bioorg Med Chem Lett. 2023 Nov 15;96:129532. doi: 10.1016/j.bmcl.2023.129532. Epub 2023 Oct 20. Bioorg Med Chem Lett. 2023. PMID: 37866714
-
Indoleamine 2, 3-dioxygenase 1 inhibitory compounds from natural sources.Front Pharmacol. 2022 Nov 4;13:1046818. doi: 10.3389/fphar.2022.1046818. eCollection 2022. Front Pharmacol. 2022. PMID: 36408235 Free PMC article. Review.
Cited by
-
Deciphering Tryptophan Oxygenation: Key Modulators of 2-Oxindole Formation in MarE.Angew Chem Int Ed Engl. 2025 Aug 25;64(35):e202510848. doi: 10.1002/anie.202510848. Epub 2025 Jul 20. Angew Chem Int Ed Engl. 2025. PMID: 40555689 Free PMC article.
References
-
- Kotake Y. Hoppe-Seyler's Z. Physiol. Chem. 1936;243:237–265. doi: 10.1515/bchm2.1936.243.6.237. - DOI
-
- Itagaki C. Nakayama Y. Hoppe-Seyler's Z. Physiol. Chem. 1941;270:83–88.
LinkOut - more resources
Full Text Sources
Research Materials

