Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study
- PMID: 34977602
- PMCID: PMC8700278
- DOI: 10.1016/S2665-9913(21)00394-5
Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study
Erratum in
-
Correction to Lancet Rheumatol 2022; 4: e177-87.Lancet Rheumatol. 2024 Jun;6(6):e336. doi: 10.1016/S2665-9913(24)00114-0. Epub 2024 Apr 20. Lancet Rheumatol. 2024. PMID: 38648816 Free PMC article. No abstract available.
Abstract
Background: In rituximab-treated patients with rheumatoid arthritis, humoral and cellular immune responses after two or three doses of SARS-CoV-2 vaccines are not well characterised. We aimed to address this knowledge gap.
Methods: This prospective, cohort study (Nor-vaC) was done at two hospitals in Norway. For this sub-study, we enrolled patients with rheumatoid arthritis on rituximab treatment and healthy controls who received SARS-CoV-2 vaccines according to the Norwegian national vaccination programme. Patients with insufficient serological responses to two doses (antibody to the receptor-binding domain [RBD] of the SARS-CoV-2 spike protein concentration <100 arbitrary units [AU]/mL) were allotted a third vaccine dose. Antibodies to the RBD of the SARS-CoV-2 spike protein were measured in serum 2-4 weeks after the second and third doses. Vaccine-elicited T-cell responses were assessed in vitro using blood samples taken before and 7-10 days after the second dose and 3 weeks after the third dose from a subset of patients by stimulating cryopreserved peripheral blood mononuclear cells with spike protein peptides. The main outcomes were the proportions of participants with serological responses (anti-RBD antibody concentrations of ≥70 AU/mL) and T-cell responses to spike peptides following two and three doses of SARS-CoV-2 vaccines. The study is registered at ClinicalTrials.gov, NCT04798625, and is ongoing.
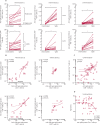
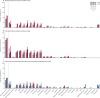
Findings: Between Feb 9, 2021, and May 27, 2021, 90 patients were enrolled, 87 of whom donated serum and were included in our analyses (69 [79·3%] women and 18 [20·7%] men). 1114 healthy controls were included (854 [76·7%] women and 260 [23·3%] men). 49 patients were allotted a third vaccine dose. 19 (21·8%) of 87 patients, compared with 1096 (98·4%) of 1114 healthy controls, had a serological response after two doses (p<0·0001). Time since last rituximab infusion (median 267 days [IQR 222-324] in responders vs 107 days [80-152] in non-responders) and vaccine type (mRNA-1273 vs BNT162b2) were significantly associated with serological response (adjusting for age and sex). After two doses, 10 (53%) of 19 patients had CD4+ T-cell responses and 14 (74%) had CD8+ T-cell responses. A third vaccine dose induced serological responses in eight (16·3%) of 49 patients, but induced CD4+ and CD8+ T-cell responses in all patients assessed (n=12), including responses to the SARS-CoV-2 delta variant (B.1.617.2). Adverse events were reported in 32 (48%) of 67 patients and in 191 (78%) of 244 healthy controls after two doses, with the frequency not increasing after the third dose. There were no serious adverse events or deaths.
Interpretation: This study provides important insight into the divergent humoral and cellular responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis. A third vaccine dose given 6-9 months after a rituximab infusion might not induce a serological response, but could be considered to boost the cellular immune response.
Funding: The Coalition for Epidemic Preparedness Innovations, Research Council of Norway Covid, the KG Jebsen Foundation, Oslo University Hospital, the University of Oslo, the South-Eastern Norway Regional Health Authority, Dr Trygve Gythfeldt og frues forskningsfond, the Karin Fossum Foundation, and the Research Foundation at Diakonhjemmet Hospital.
© 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
KKJ reports speakers bureaus from Roche and BMS and advisory board participation for Celltrion and Norgine. JJ reports grants from Abbvie, Pharmacosmos, and Ferring; consulting fees from Abbvie, Boerhinger Ingelheim, BMS, Celltrion, Ferring, Glihead, Janssen Cilag, MSD, Napp Pharma, Novartis, Orion Pharma, Pfeizer, Pharmacosmos, Takeda, Sandoz, and Unimedic Pharma; and speakers bureaus from Abbvie, Astro Pharma, Boerhinger Ingelheim, BMS, Celltrion, Ferring, Glihead, Hikma, Janssen Cilag, Meda, MSD, Napp Pharma, Novartis, Oriuon Pharma, Pfeizer, Pharmacosmos, Roche, Takeda, and Sandoz. TKK reports grants from AbbVie, Amgen, BMS, MSD, Novartis, Pfizer, and UCB; consulting fees from AbbVie, Amgen, Biogen, Celltrion, Eli Lilly, Gilead, Mylan, Novartis, Pfizer, Sandoz, and Sanofi; speakers bureaus from Amgen, Celltrion, Egis, Evapharma, Ewopharma, Hikma, Oktal, Sandoz, and Sanofi; and participation on a data safety monitoring board for AbbVie. LAM reports funding from the KG Jebsen foundation; support for infrastructure and biobanking from the University of Oslo and Oslo University Hospital; grants from the Coalition of Epidemic Preparedness Innovations (CEPI); and speakers bureaus from Novartis and Cellgene. GG reports consulting fees from the Norwegian System of Compensation to Patients and AstraZeneca, and speakers bureaus from Bayer, Sanofi Pasteur, and Thermo Fisher. JTV reports grant from the CEPI. FL-J reports grants from the CEPI and the South-Eastern Norway Regional Health Authority. GLG reports funding from the Karin Fossum foundation, Diakonhjemmet Hospital, Oslo University Hospital, Akershus University Hospital, the Dr Trygve Gydtfeldt og frues Foundation, and the South-Eastern Norway Regional Health Authority; consulting fees from AbbVie and Pfizer; speakers fees from AbbVie, Pfizer, Sandoz, Orion Pharma, Novartis, and UCB; and advisory board participation for Pfizer and AbbVie. All other authors declare no competing interests.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

