Therapeutic advances in non-small cell lung cancer: Focus on clinical development of targeted therapy and immunotherapy
- PMID: 34977873
- PMCID: PMC8706764
- DOI: 10.1002/mco2.105
Therapeutic advances in non-small cell lung cancer: Focus on clinical development of targeted therapy and immunotherapy
Abstract
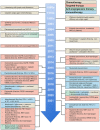
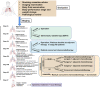
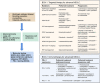
Lung cancer still contributes to nearly one-quarter cancer-related deaths in the past decades, despite the rapid development of targeted therapy and immunotherapy in non-small cell lung cancer (NSCLC). The development and availability of comprehensive genomic profiling make the classification of NSCLC more precise and personalized. Most treatment decisions of advanced-stage NSCLC have been made based on the genetic features and PD-L1 expression of patients. For the past 2 years, more than 10 therapeutic strategies have been approved as first-line treatment for certain subgroups of NSCLC. However, some major challenges remain, including drug resistance and low rate of overall survival. Therefore, we discuss and review the therapeutic strategies of NSCLC, and focus on the development of targeted therapy and immunotherapy in advanced-stage NSCLC. Based on the latest guidelines, we provide an updated summary on the standard treatment for NSCLC. At last, we discussed several potential therapies for NSCLC. The development of new drugs and combination therapies both provide promising therapeutic effects on NSCLC.
Keywords: combination therapy; drug resistance; immunotherapy; non‐small cell lung cancer (NSCLC); targeted therapy.
© 2021 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. - PubMed
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. - PubMed
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Martín‐Sánchez JC, Lunet N, González‐Marrón A, et al. Projections in breast and lung cancer mortality among women: a Bayesian analysis of 52 countries worldwide. Cancer Res. 2018;78(15):4436‐4442. - PubMed
-
- Smith CJ, Perfetti TA, Rumple MA, Rodgman A, Doolittle DJ. “IARC Group 2A Carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol. 2000;38(4):371‐383. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
