Janus kinase 1 inhibitor INCB054707 for patients with moderate-to-severe hidradenitis suppurativa: results from two phase II studies
- PMID: 34978076
- PMCID: PMC9314604
- DOI: 10.1111/bjd.20969
Janus kinase 1 inhibitor INCB054707 for patients with moderate-to-severe hidradenitis suppurativa: results from two phase II studies
Abstract
Background: Janus kinase (JAK)-mediated cytokine signalling contributes to local and systemic inflammation in hidradenitis suppurativa (HS).
Objectives: To describe the safety and efficacy results from two multicentre phase II trials of the JAK1 inhibitor INCB054707 in patients with moderate-to-severe HS.
Methods: Patients received open-label INCB054707 15 mg once daily (QD; Study 1) or were randomized to INCB054707 30, 60 or 90 mg QD or placebo (3 : 1 within each cohort; Study 2) for 8 weeks. Eligible patients were aged 18-75 years and had moderate-to-severe HS (Hurley stage II/III disease), lesions present in at least two anatomical locations, and a total abscess and inflammatory nodule count ≥ 3. The primary endpoint for both studies was safety and tolerability. Secondary endpoints included HS Clinical Response (HiSCR) and other efficacy measures.
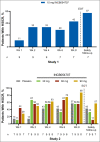
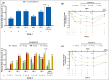
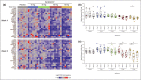
Results: Ten patients were enrolled in Study 1 (15 mg INCB054707) and 35 in Study 2 (INCB054707: 30 mg, n = 9; 60 mg, n = 9; 90 mg, n = 8; placebo, n = 9). Overall, 70% of patients in Study 1 and 81% of patients receiving INCB054707 in Study 2 experienced at least one treatment-emergent adverse event; 30% and 42% of patients, respectively, had at least one treatment-related adverse event. Among the evaluable patients, three (43%) in Study 1 and 17 (65% overall: 30 mg, 56%; 60 mg, 56%; 90 mg, 88%) receiving INCB054707 vs. 4 patients (57%) receiving placebo in Study 2 achieved HiSCR at week 8.
Conclusions: INCB054707 was well tolerated, with responses observed in patients with moderate-to-severe HS. The safety and efficacy findings from these studies demonstrate proof of concept for JAK1 inhibition in HS. The studies are registered on ClinicalTrials.gov (NCT03569371 and NCT03607487).
© 2022 Incyte Corporation. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures

Comment in
-
The impact of Janus kinase 1 inhibitors on quality of life in patients with hidradenitis suppurativa.Br J Dermatol. 2022 Aug;187(2):274. doi: 10.1111/bjd.21045. Epub 2022 May 3. Br J Dermatol. 2022. PMID: 35122235 No abstract available.
-
Janus kinase inhibitors for hidradenitis suppurativa: expanding the therapeutic toolbox.Br J Dermatol. 2022 May;186(5):768-769. doi: 10.1111/bjd.21297. Epub 2022 Apr 12. Br J Dermatol. 2022. PMID: 35415896 No abstract available.
Similar articles
-
Efficacy and safety of the oral Janus kinase 1 inhibitor povorcitinib (INCB054707) in patients with hidradenitis suppurativa in a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study.J Am Acad Dermatol. 2024 Mar;90(3):521-529. doi: 10.1016/j.jaad.2023.10.034. Epub 2023 Oct 21. J Am Acad Dermatol. 2024. PMID: 37871805 Clinical Trial.
-
Long-term efficacy and safety of secukinumab in patients with moderate-to-severe hidradenitis suppurativa: week 104 results from the SUNSHINE and SUNRISE extension trial.Br J Dermatol. 2025 Mar 18;192(4):629-640. doi: 10.1093/bjd/ljae469. Br J Dermatol. 2025. PMID: 39611771 Clinical Trial.
-
Proof-of-concept study exploring the effect of spesolimab in patients with moderate-to-severe hidradenitis suppurativa: a randomized double-blind placebo-controlled clinical trial.Br J Dermatol. 2024 Sep 18;191(4):508-518. doi: 10.1093/bjd/ljae144. Br J Dermatol. 2024. PMID: 38576350 Clinical Trial.
-
The use of biologics and JAK inhibitors in the management of moderate to severe Hidradenitis Suppurativa treatment: a scoping review.Arch Dermatol Res. 2024 May 25;316(6):259. doi: 10.1007/s00403-024-03121-x. Arch Dermatol Res. 2024. PMID: 38795234
-
Adalimumab: A Review in Hidradenitis Suppurativa.Am J Clin Dermatol. 2016 Oct;17(5):545-552. doi: 10.1007/s40257-016-0220-6. Am J Clin Dermatol. 2016. PMID: 27665300 Review.
Cited by
-
Hidradenitis Suppurativa: New Targets and Emerging Treatments.Am J Clin Dermatol. 2024 Sep;25(5):765-778. doi: 10.1007/s40257-024-00880-1. Epub 2024 Jul 26. Am J Clin Dermatol. 2024. PMID: 39060744 Review.
-
[Janus kinase inhibitors for skin disorders].Dermatologie (Heidelb). 2024 Oct;75(10):781-790. doi: 10.1007/s00105-024-05406-8. Epub 2024 Aug 30. Dermatologie (Heidelb). 2024. PMID: 39212722 German.
-
Efficacy and Safety of Biologics and Small Molecules for Moderate-to-Severe Hidradenitis Suppurativa: A Systematic Review and Network Meta-Analysis.Pharmaceutics. 2023 Apr 28;15(5):1351. doi: 10.3390/pharmaceutics15051351. Pharmaceutics. 2023. PMID: 37242593 Free PMC article. Review.
-
Improvement of Recalcitrant Dissecting Cellulitis of the Scalp After a Trial of Upadacitinib.Cureus. 2024 Jan 16;16(1):e52377. doi: 10.7759/cureus.52377. eCollection 2024 Jan. Cureus. 2024. PMID: 38361718 Free PMC article.
-
The Importance of the Pyrazole Scaffold in the Design of Protein Kinases Inhibitors as Targeted Anticancer Therapies.Molecules. 2023 Jul 12;28(14):5359. doi: 10.3390/molecules28145359. Molecules. 2023. PMID: 37513232 Free PMC article. Review.
References
-
- Sabat R, Jemec GBE, Matusiak L et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020; 6:18. - PubMed
-
- Nguyen TV, Damiani G, Orenstein LAV et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol 2021; 35:50–61. - PubMed
-
- Ingram JR, Jenkins‐Jones S, Knipe DW et al. Population‐based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018; 178:917–24. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

