C4-Dicarboxylates as Growth Substrates and Signaling Molecules for Commensal and Pathogenic Enteric Bacteria in Mammalian Intestine
- PMID: 34978458
- PMCID: PMC9017328
- DOI: 10.1128/JB.00545-21
C4-Dicarboxylates as Growth Substrates and Signaling Molecules for Commensal and Pathogenic Enteric Bacteria in Mammalian Intestine
Abstract
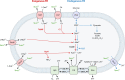
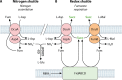
The C4-dicarboxylates (C4-DC) l-aspartate and l-malate have been identified as playing an important role in the colonization of mammalian intestine by enteric bacteria, such as Escherichia coli and Salmonella enterica serovar Typhimurium, and succinate as a signaling molecule for host-enteric bacterium interaction. Thus, endogenous and exogenous fumarate respiration and related functions are required for efficient initial growth of the bacteria. l-Aspartate represents a major substrate for fumarate respiration in the intestine and a high-quality substrate for nitrogen assimilation. During nitrogen assimilation, DcuA catalyzes an l-aspartate/fumarate antiport and serves as a nitrogen shuttle for the net uptake of ammonium only, whereas DcuB acts as a redox shuttle that catalyzes the l-malate/succinate antiport during fumarate respiration. The C4-DC two-component system DcuS-DcuR is active in the intestine and responds to intestinal C4-DC levels. Moreover, in macrophages and in mice, succinate is a signal that promotes virulence and survival of S. Typhimurium and pathogenic E. coli. On the other hand, intestinal succinate is an important signaling molecule for the host and activates response and protective programs. Therefore, C4-DCs play a major role in supporting colonization of enteric bacteria and as signaling molecules for the adaptation of host physiology.
Keywords: C4-dicarboxylates; Escherichia coli; Salmonella; Salmonella Typhimurium; fumarate respiration; initial growth; intestine colonization; l-aspartate; nitrogen assimilation; succinate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

