Inhibiting pyruvate kinase muscle isoform 2 regresses group 2 pulmonary hypertension induced by supra-coronary aortic banding
- PMID: 34978755
- PMCID: PMC8810721
- DOI: 10.1111/apha.13764
Inhibiting pyruvate kinase muscle isoform 2 regresses group 2 pulmonary hypertension induced by supra-coronary aortic banding
Abstract
Introduction: Group 2 pulmonary hypertension (PH) has no approved PH-targeted therapy. Metabolic remodelling, specifically a biventricular increase in pyruvate kinase muscle (PKM) isozyme 2 to 1 ratio, occurs in rats with group 2 PH induced by supra-coronary aortic banding (SAB). We hypothesize that increased PKM2/PKM1 is maladaptive and inhibiting PKM2 would improve right ventricular (RV) function.
Methods: Male, Sprague-Dawley SAB rats were confirmed to have PH by echocardiography and then randomized to treatment with a PKM2 inhibitor (intraperitoneal shikonin, 2 mg/kg/day) versus 5% DMSO (n = 5/group) or small interfering RNA-targeting PKM2 (siPKM2) versus siRNA controls (n = 7/group) by airway nebulization.
Results: Shikonin-treated SAB rats had milder PH (PAAT 32.1 ± 1.3 vs 22.1 ± 1.2 ms, P = .0009) and lower RV systolic pressure (RVSP) (31.5 ± 0.9 vs 55.7 ± 1.9 mm Hg, P < .0001) versus DMSO-SAB rats. siPKM2 nebulization reduced PKM2 expression in the RV, increased PAAT (31.7 ± 0.7 vs 28.0 ± 1.3 ms, P = .025), lowered RVSP (30.6 ± 2.6 vs 42.0 ± 4.0 mm Hg, P = .032) and reduced diastolic RVFW thickness (0.69 ± 0.04 vs 0.85 ± 0.06 mm, P = .046). Both shikonin and siPKM2 regressed PH-induced medial hypertrophy of small pulmonary arteries.
Conclusion: Increases in PKM2/PKM1 in the RV contribute to RV dysfunction in group 2 PH. Chemical or molecular inhibition of PKM2 restores the normal PKM2/PKM1 ratio, reduces PH, RVSP and RVH and regresses adverse PA remodelling. PKM2 merits consideration as a therapeutic cardiac target for group 2 PH.
Keywords: heart failure with preserved ejection fraction; left ventricular hypertrophy; pyruvate kinase muscle isoform 2; right ventricular hypertrophy aortic stenosis; shikonin; uncoupled glycolysis.
© 2022 Scandinavian Physiological Society. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

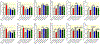
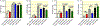


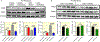


References
-
- Khush KK, Tasissa G, Butler J, McGlothlin D, De Marco T and Investigators E. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) database. Am Heart J. 2009;157:1026–34. - PubMed
-
- Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P and Rich S. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–65. - PubMed
-
- Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, Barber D, Johnson A, Doliszny KM and Archer SL. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension-A Population-Based Cohort Study in Ontario, Canada. Circulation: Cardiovascular Quality and Outcomes. 2018;11:e003973. - PMC - PubMed

