Exciton Delocalization in a DNA-Templated Organic Semiconductor Dimer Assembly
- PMID: 34979076
- PMCID: PMC8793135
- DOI: 10.1021/acsnano.1c09143
Exciton Delocalization in a DNA-Templated Organic Semiconductor Dimer Assembly
Abstract
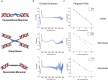
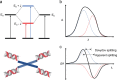
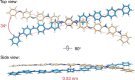
A chiral dimer of an organic semiconductor was assembled from octaniline (octamer of polyaniline) conjugated to DNA. Facile reconfiguration between the monomer and dimer of octaniline-DNA was achieved. The geometry of the dimer and the exciton coupling between octaniline molecules in the assembly was studied both experimentally and theoretically. The octaniline dimer was readily switched between different electronic states by protonic doping and exhibited a Davydov splitting comparable to those previously reported for DNA-dye systems employing dyes with strong transition dipoles. This approach provides a possible platform for studying the fundamental properties of organic semiconductors with DNA-templated assemblies, which serve as candidates for artificial light-harvesting systems and excitonic devices.
Keywords: DNA conjugation; circular dichroism; exciton delocalization; organic semiconductor; proton doping.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Exciton delocalization in a fully synthetic DNA-templated bacteriochlorin dimer.Phys Chem Chem Phys. 2023 Oct 25;25(41):28437-28451. doi: 10.1039/d3cp01634j. Phys Chem Chem Phys. 2023. PMID: 37843877 Free PMC article.
-
Exciton Delocalization in Indolenine Squaraine Aggregates Templated by DNA Holliday Junction Scaffolds.J Phys Chem B. 2020 Oct 29;124(43):9636-9647. doi: 10.1021/acs.jpcb.0c06480. Epub 2020 Oct 14. J Phys Chem B. 2020. PMID: 33052691 Free PMC article.
-
An Organic Semiconductor Organized into 3D DNA Arrays by "Bottom-up" Rational Design.Angew Chem Int Ed Engl. 2017 Jun 1;56(23):6445-6448. doi: 10.1002/anie.201700462. Epub 2017 May 3. Angew Chem Int Ed Engl. 2017. PMID: 28466984
-
Keeping the chromophores crossed: evidence for null exciton splitting.Chem Soc Rev. 2023 Oct 2;52(19):6664-6679. doi: 10.1039/d3cs00176h. Chem Soc Rev. 2023. PMID: 37606527 Review.
-
Dynamics of Excitons in Conjugated Molecules and Organic Semiconductor Systems.Chem Rev. 2022 May 11;122(9):8487-8593. doi: 10.1021/acs.chemrev.1c00648. Epub 2022 Mar 17. Chem Rev. 2022. PMID: 35298145 Review.
Cited by
-
Exciton Chirality Inversion in Dye Dimers Templated by DNA Holliday Junction.J Phys Chem Lett. 2022 Nov 24;13(46):10688-10696. doi: 10.1021/acs.jpclett.2c02721. Epub 2022 Nov 10. J Phys Chem Lett. 2022. PMID: 36355575 Free PMC article.
-
Exciton delocalization in a fully synthetic DNA-templated bacteriochlorin dimer.Phys Chem Chem Phys. 2023 Oct 25;25(41):28437-28451. doi: 10.1039/d3cp01634j. Phys Chem Chem Phys. 2023. PMID: 37843877 Free PMC article.
-
Supramolecular assembly of pyrene-DNA conjugates: influence of pyrene substitution pattern and implications for artificial LHCs.Org Biomol Chem. 2023 Oct 11;21(39):7908-7912. doi: 10.1039/d3ob01375h. Org Biomol Chem. 2023. PMID: 37750811 Free PMC article.
-
Towards control of excitonic coupling in DNA-templated Cy5 aggregates: the principal role of chemical substituent hydrophobicity and steric interactions.Nanoscale. 2023 Feb 16;15(7):3284-3299. doi: 10.1039/d2nr05544a. Nanoscale. 2023. PMID: 36723027 Free PMC article.
-
Pursuing excitonic energy transfer with programmable DNA-based optical breadboards.Chem Soc Rev. 2023 Nov 13;52(22):7848-7948. doi: 10.1039/d0cs00936a. Chem Soc Rev. 2023. PMID: 37872857 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

