IL-17 crosses the blood-brain barrier to trigger neuroinflammation: a novel mechanism in nitroglycerin-induced chronic migraine
- PMID: 34979902
- PMCID: PMC8903553
- DOI: 10.1186/s10194-021-01374-9
IL-17 crosses the blood-brain barrier to trigger neuroinflammation: a novel mechanism in nitroglycerin-induced chronic migraine
Abstract
Background: Chronic migraine places a disabling burden on patients, which is extensively modeled by the nitroglycerin (NTG)-treated animal model. Although the NF-κB pathway is involved in an increase in CGRP levels and activation of the trigeminal system in the NTG model, the relationship between NTG and neuroinflammation remains unclear. This study aimed to optimize a chronic NTG rat model with hyperalgesia and the ethological capacity for estimating migraine therapies and to further explore the underlying mechanism of NTG-induced migraine.
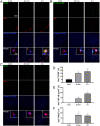
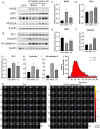
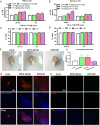
Methods: Rats were administered different doses of NTG s.c. daily or every 2 d; 30 min and 2 h later, the mechanical threshold was tested. After 9 d, the rats were injected with EB or Cy5.5 for the permeability assay. The other animals were sacrificed, and then, brainstem and caudal trigeminal ganglion were removed to test CGRP, c-Fos and NOS activity; Cytokines levels in the tissue and serum were measured by ELISA; and NF-κB pathway and blood-brain barrier (BBB)-related indicators were analyzed using western blotting. Immunohistochemistry was performed to observe microglial polarization and IL-17A+ T cell migration in the medulla oblongata.
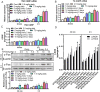
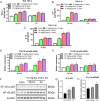
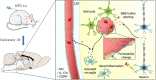
Results: NTG (10 mg/kg, s.c., every 2 d for a total of 5 injections) was the optimal condition, resulting in progressive hyperalgesia and migraine behavior. TNC neuroinflammation with increases in cytokines, CGRP and c-Fos and activation of the NF-κB pathway was observed, and these changes were alleviated by ibuprofen. Furthermore, NTG administration increased BBB permeability by altering the levels functional proteins (RAGE, LRP1, AQP4 and MFSD2A) and structural proteins (ZO-1, Occludin and VE-cadherin-2) to increase peripheral IL-17A permeation into the medulla oblongata, activating microglia and neuroinflammation, and eventually causing hyperalgesia and migraine attack.
Conclusions: This study confirmed that NTG (10 mg/kg, s.c., every 2 d for a total of 5 injections) was the optimal condition to provoke migraine, resulting in mechanical hyperalgesia and observable migraine-like behavior. Furthermore, IL-17A crossed the blood-brain barrier into the medulla oblongata, triggering TNC activation through microglia-mediated neuroinflammation. This process was a novel mechanism in NTG-induced chronic migraine, suggesting that IL-17A might be a novel target in the treatment of migraine.
Keywords: Blood–brain barrier; IL-17; Migraine; Neuroinflammation; Nitroglycerin.
© 2021. The Author(s).
Conflict of interest statement
The authors have no conflict of interest exits regarding the contents of the manuscript.
Figures




References
-
- Albrecht DS, Mainero C, Ichijo E, Ward N, Granziera C, Zürcher NR, Akeju O, Bonnier G, Price J, Hooker JM, Napadow V, Loggia ML, Hadjikhani N. Imaging of neuroinflammation in migraine with aura: a [(11) C]PBR28 PET/MRI study. Neurology. 2019;92(17):e2038–e2050. doi: 10.1212/wnl.0000000000007371. - DOI - PMC - PubMed
-
- Alli S, Figueiredo CA, Golbourn B, Sabha N, Wu MY, Bondoc A, Luck A, Coluccia D, Maslink C, Smith C, Wurdak H, Hynynen K, O'Reilly M, Rutka JT. Brainstem blood brain barrier disruption using focused ultrasound: a demonstration of feasibility and enhanced doxorubicin delivery. J Control Release. 2018;281:29–41. doi: 10.1016/j.jconrel.2018.05.005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

